Author: Lou Deda
Organization: Shenzhen Chuangxin Medical Technology Co., Ltd.

Background and Treatment Needs
Lou Deda highlights the high mortality rate associated with untreated Type A aortic dissection, which is particularly critical in the initial days following onset. He notes that while Type B dissections have commercially available intraluminal treatments, Type A dissections are primarily treated surgically, with no commercial products yet available for intraluminal treatment.

Product Design and Innovation
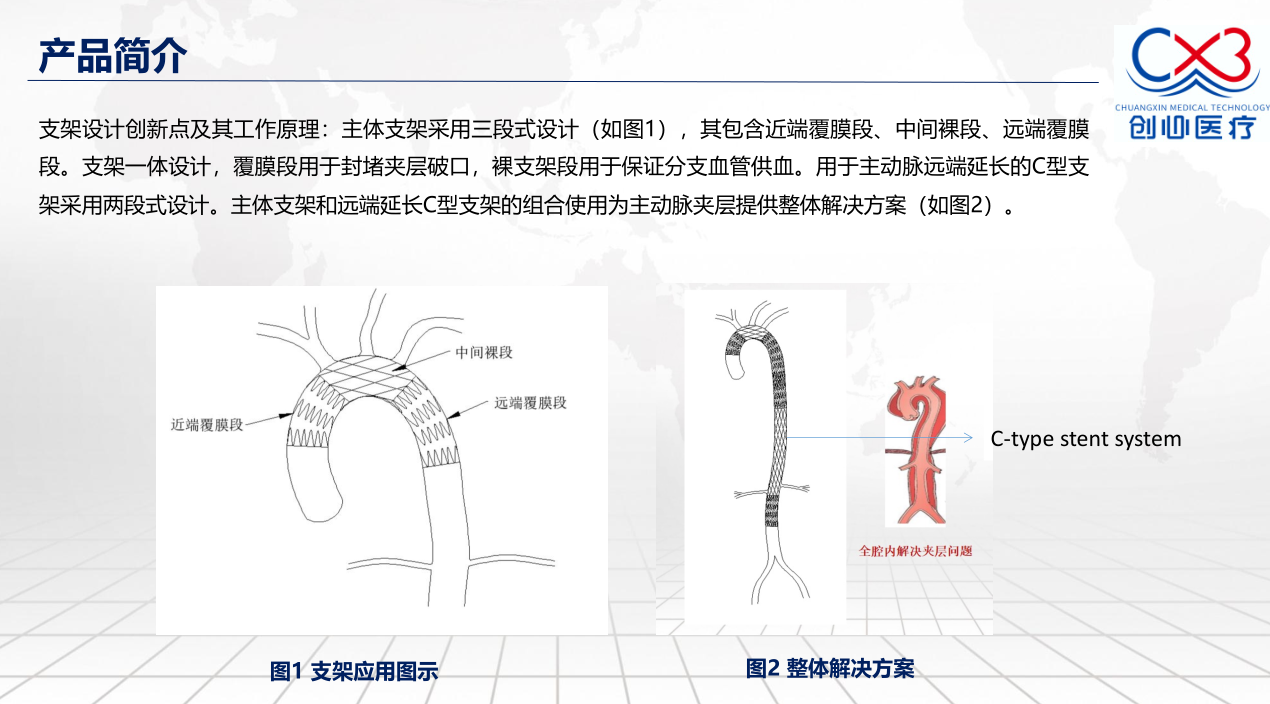
• Stent Structure: Features a three-part design including a proximal covered segment, a middle bare segment, and a distal covered segment. This design aims to seal the dissection entry tear and ensure blood supply to branch vessels.
• Technical Innovation: The delivery system of the stent includes an adjustable bend design to enhance safety when navigating through curved vascular segments. The stent is specifically designed to be flexible enough to handle 180-degree turns.

Clinical Research and Case Studies
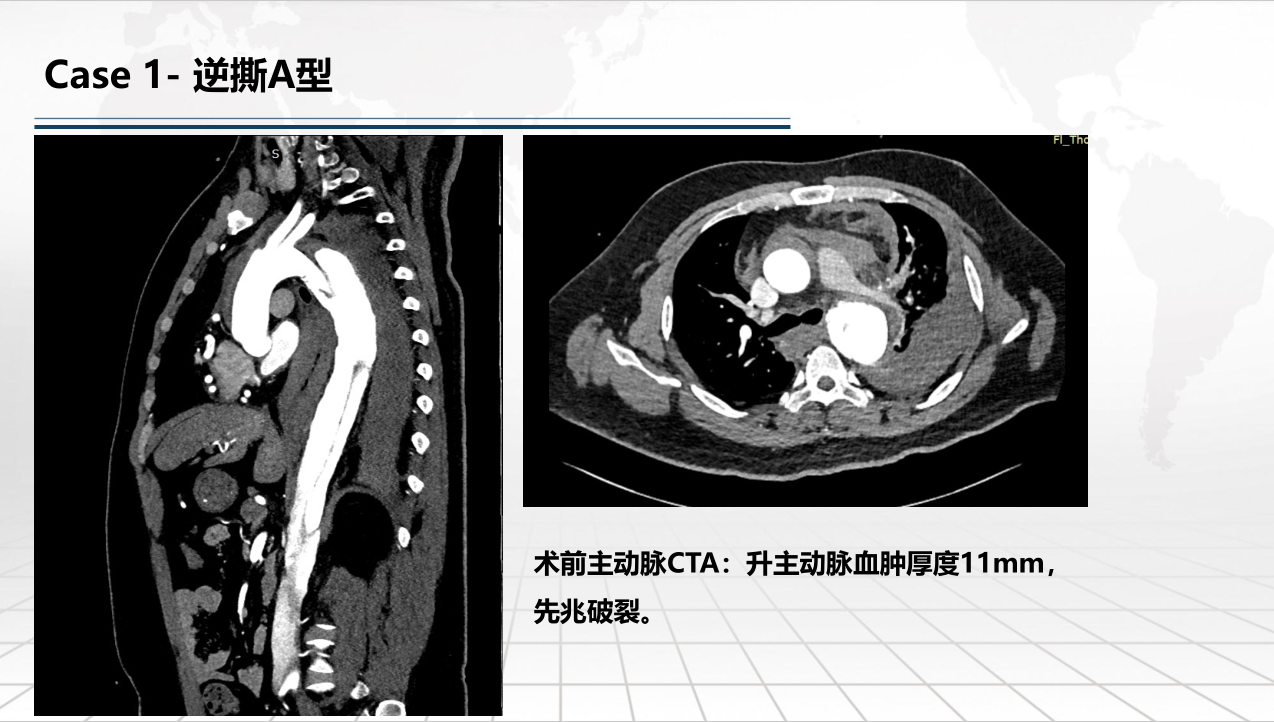
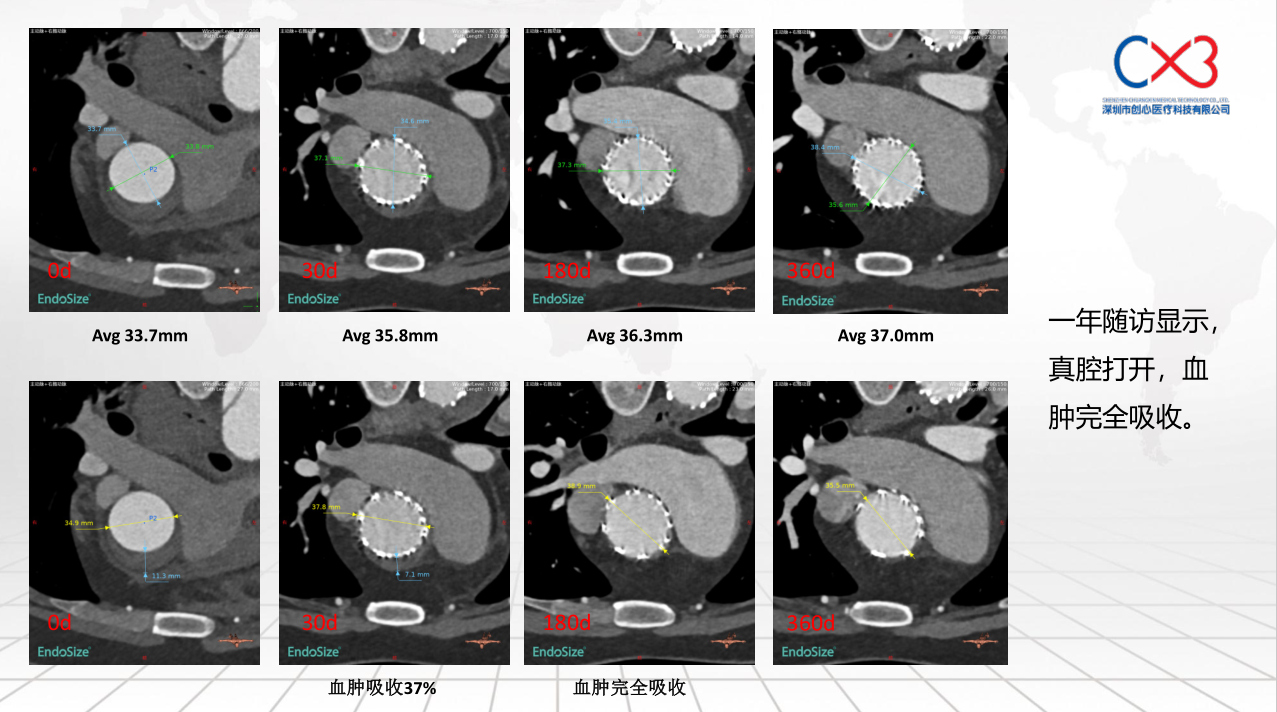
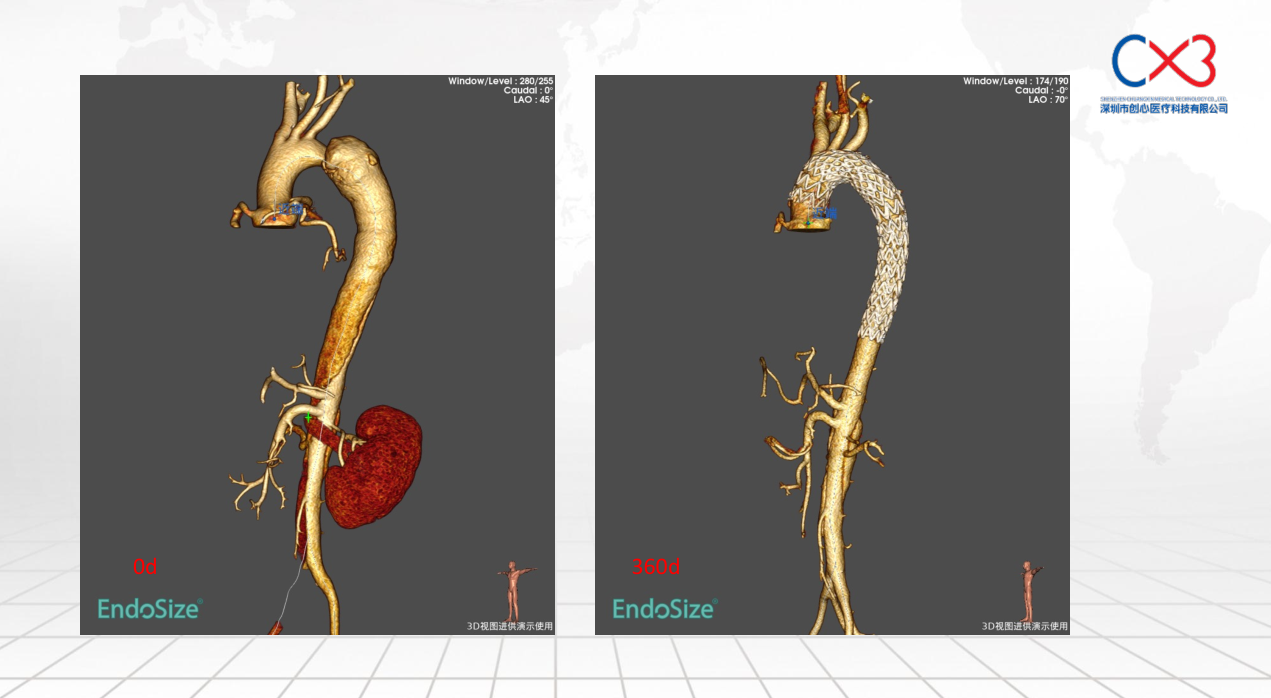
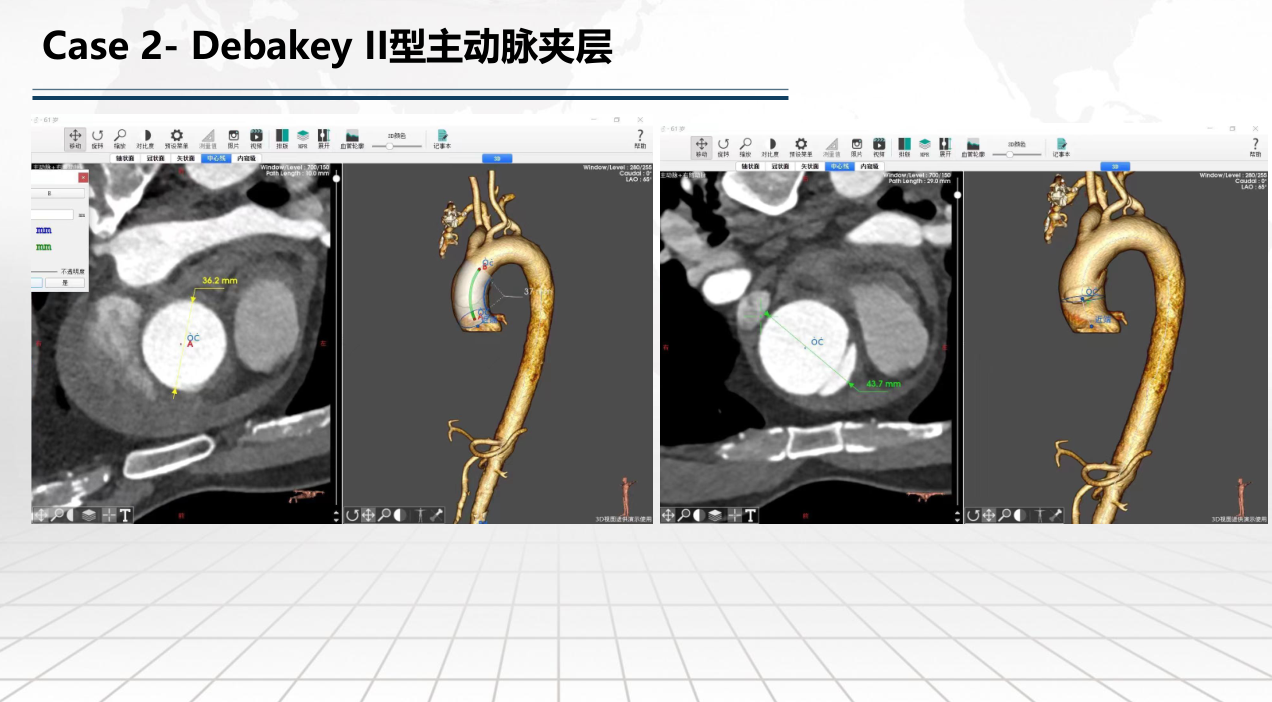
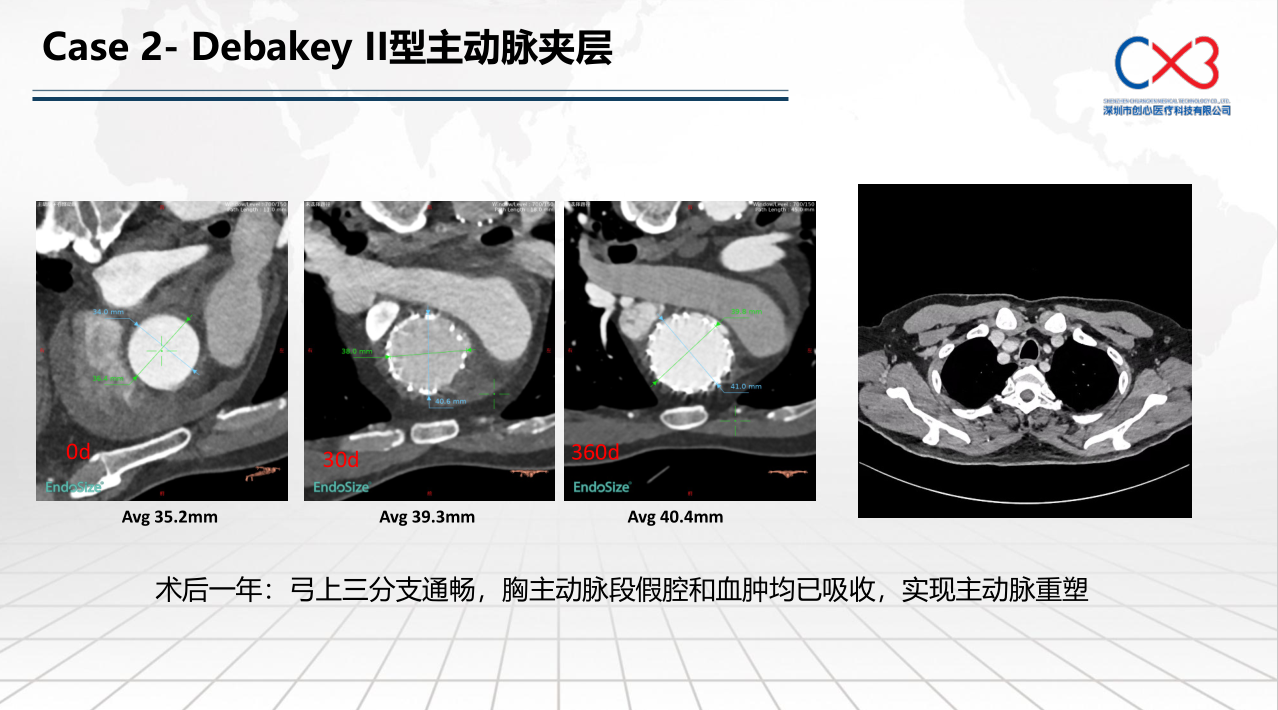
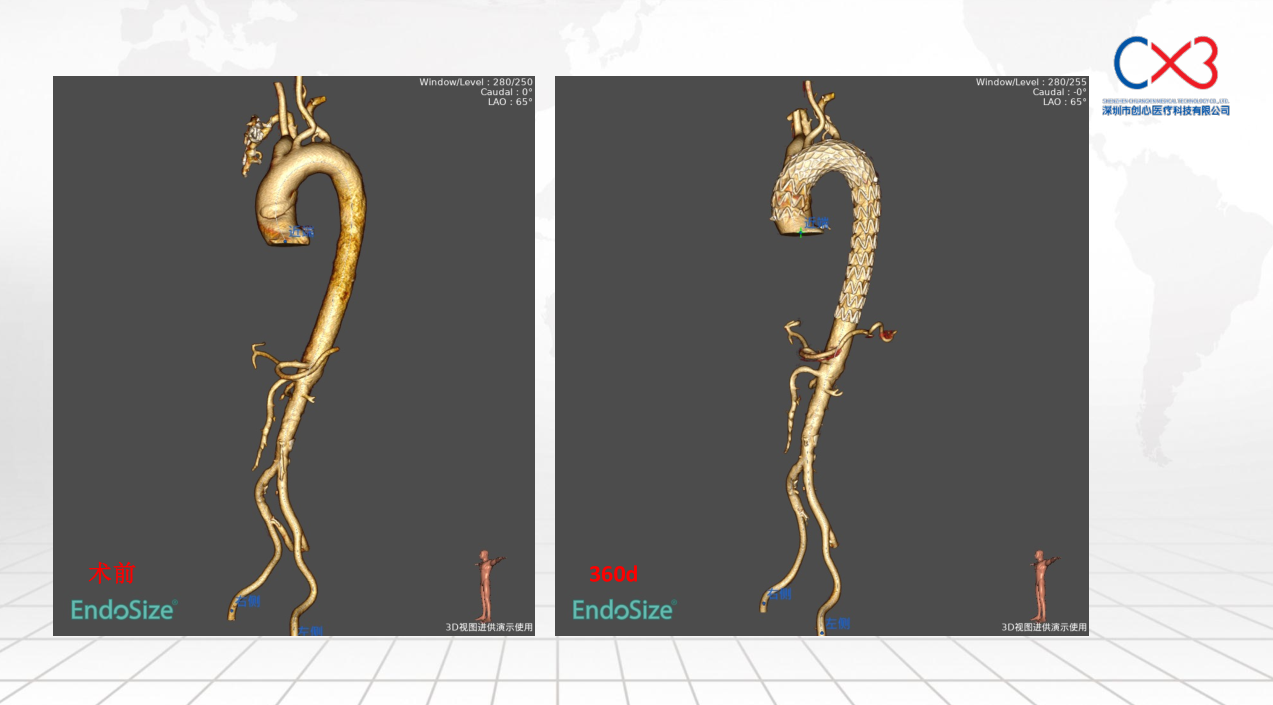
• FIM (First-In-Human) Clinical Trial Results: Outlines results from the initial clinical trials involving 9 patients, with follow-ups extending up to one year, demonstrating no deaths or strokes. Specific cases of retrograde Type A and Debakey II aortic dissections are discussed to illustrate the post-operative absorption of hematomas and aortic remodeling.
Summary and Acknowledgements
Lou Deda summarizes the preliminary validation results of the stent system, affirming its safety and effectiveness, while noting that long-term effects will require further follow-up and support from multicenter clinical trials.

Contact Us
For more in-depth discussion and exchange on the content related to the Tianfu Great Vessel Conference or any inquiries, please contact us via email at: endovascluar@simtomax.cn.
For more international information, please visit:
Let’s join forces for better health and showcase your achievements to the world!


