Organization: Jiangsu Bai You Da Life Science & Technology Co., Ltd.
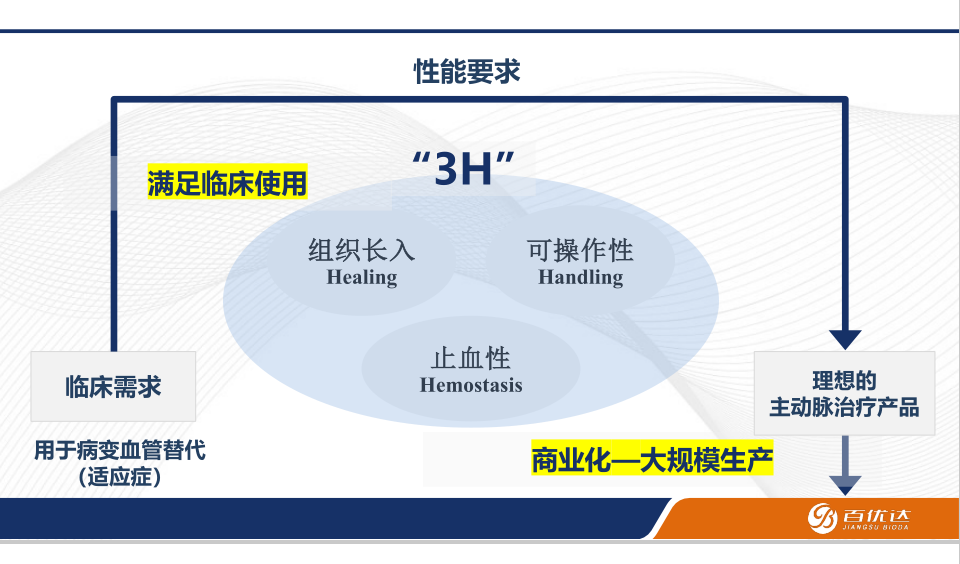
Product Design and Performance Requirements
• Material: The development team emphasized the use of PET polyester (Dacron) as the primary material, optimized for tissue ingrowth and reduced blood seepage.
• Design Features: The artificial vessels include a striped reference line to facilitate vascular anastomosis and prevent twisting.
Innovative Weaving Process
• Patented Technology: Detailed the exclusive innovative weaving technology patented by the company, featuring a satin structure on the inner and outer layers and a combination of elastic and non-elastic yarns to enhance the durability and flexibility of the artificial vessels.

Clinical Trials and Effects
• Study Details: Conducted a prospective, multicenter, randomized controlled, non-inferiority clinical study at five centers, including Capital Medical University Affiliated Beijing Anzhen Hospital.
• Results: Reported on the safety and efficacy of the artificial vessels, including postoperative survival rates and patency rates of the grafts.

Intraoperative and Postoperative Data
• Key Indicators: Presented data on intraoperative blood transfusion volume, bleeding, ICU stay duration, hospitalization time, and duration of cardiopulmonary bypass, demonstrating the performance of VASOLINE® artificial vessels in surgical settings.
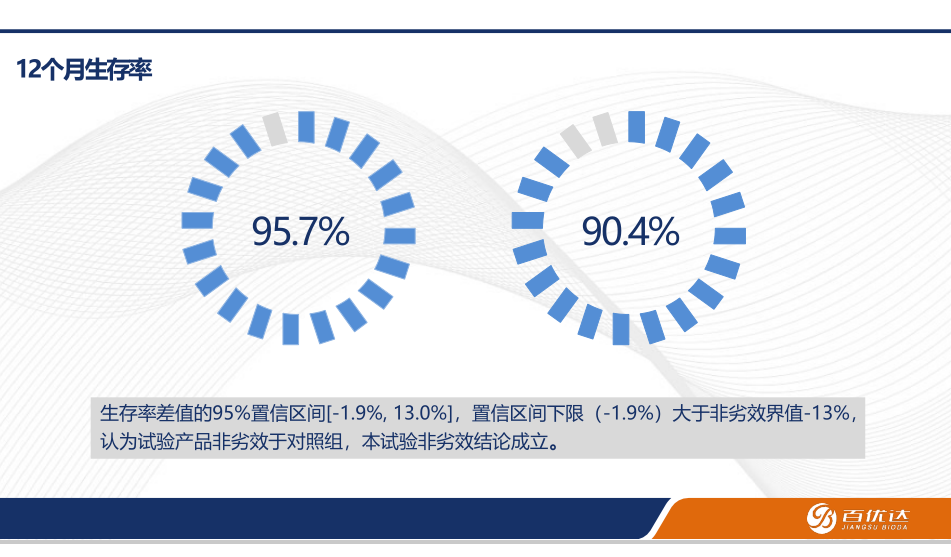
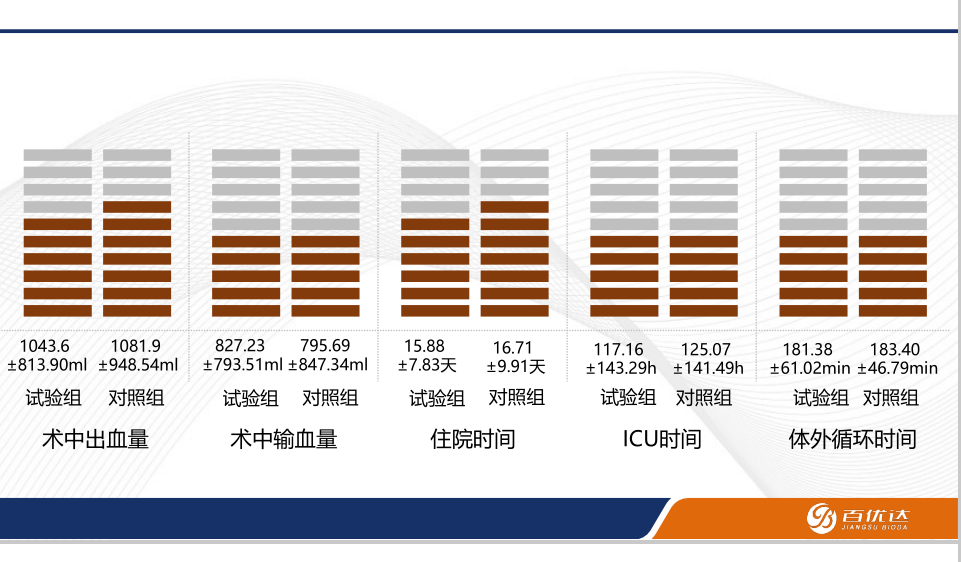
Conclusions and Future Outlook
• Clinical Application: Bai You Da highlighted the promising clinical potential of VASOLINE® artificial vessels in treating major arterial diseases and plans to continue optimizing the product and expanding the scale of clinical trials.
Contact Us
For further information or inquiries about the content discussed at the Tianfu Great Vessel Conference, please email us at: endovascluar@simtomax.cn.
For more international information, visit:
Let’s join forces for better health and showcase your achievements to the world!


