
The 2nd Tianfu Vascular Conference (TFS 2024) was successfully held on May 17-18, 2024, in Chengdu. From the pinnacle of medical science, we foresee the future of the vascular discipline, recognizing the immense responsibility and honorable mission ahead. As members of the vascular field, we are tasked with advancing the discipline and improving diagnostic and treatment standards. In the future, we will analyze the content of TFS lectures to help you understand the most cutting-edge vascular treatment experiences.
In this series of articles, we will thoroughly analyze the presentation by Professors Wang Jun and Yu Hao from the Southern Theater General Hospital of the PLA at the Tianfu Vascular Conference, discussing how to utilize the new generation of GORE® TAG® Active Control Thoracic Stent-Graft System for expanding the proximal anchoring zone.

GORE® TAG® Active Control Thoracic Stent-Graft System
In 2019, the GORE® TAG® Active Control Thoracic Stent-Graft System received FDA approval and was registered and launched in China in 2022. This system offers the following features:
• Adjustable and Controllable: It provides adjustable and controllable proximal angle adjustments and can be released in stages while maintaining continuous blood flow.
• Staged Release: The stent opens from the proximal end to the mid-diameter, allowing repositioning and tangential adjustment. It then opens from the distal end to the full diameter for distal positioning.

SURPASS Registry Study Results
In the SURPASS registry project, 62.2% of the 127 patients had the stent repositioned at the mid-diameter, and 50.4% used angle control. The study results showed:
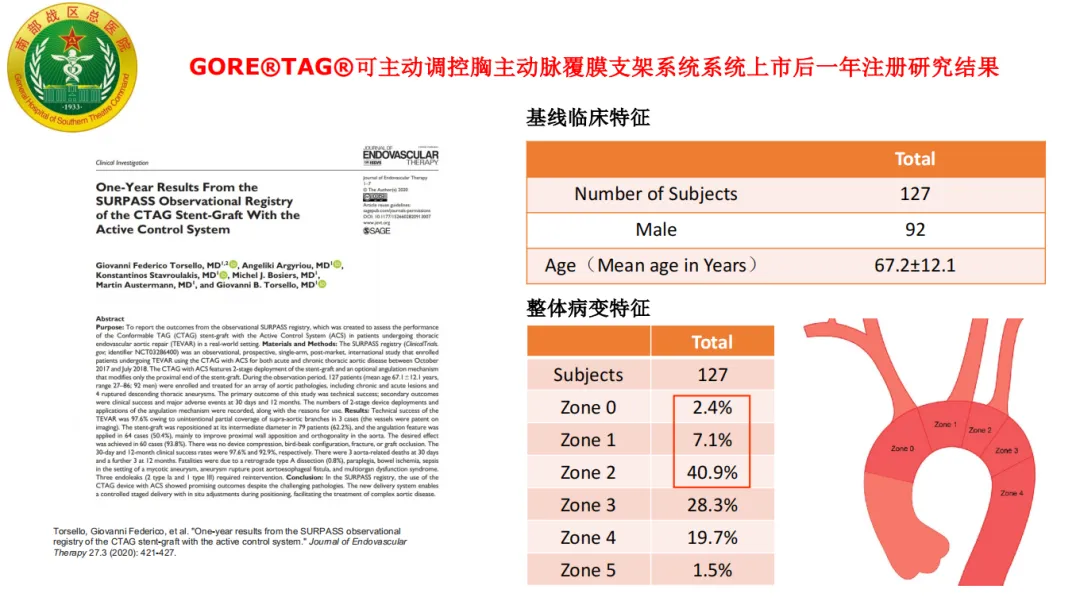
• Clinical Success Rate: The 12-month clinical success rate was 100%.
• Type I Endoleak: Three cases of Type I endoleak were reported, all managed without clinical intervention.
• False Lumen Thrombosis Rate: The rate of complete thrombosis in the false lumen of the stent segment was 90% at 12 months post-operation.
• True and False Lumen Diameter Changes: The minimum diameter of the true lumen increased by 54%, and the maximum diameter of the false lumen decreased by 82% at 12 months post-operation.
Case Studies
Case 1:
• Patient: Ms. Zhong, 59 years old
• Admission Date: January 3, 2023
• Chief Complaint: Recurrent chest and back pain for over a month
• Past Medical History: 10-year history of hypertension, 6-month history of membranous nephropathy, anxiety disorder, and mild to moderate aortic valve insufficiency
• Diagnosis: Aortic arch ulcer with intramural hematoma involving the left subclavian artery (LSA)
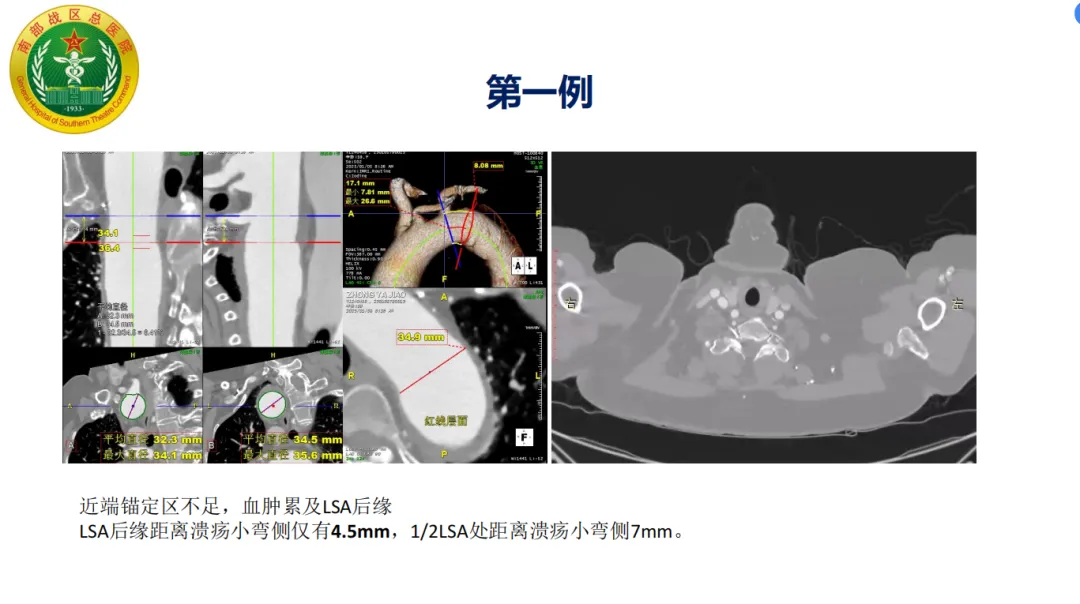
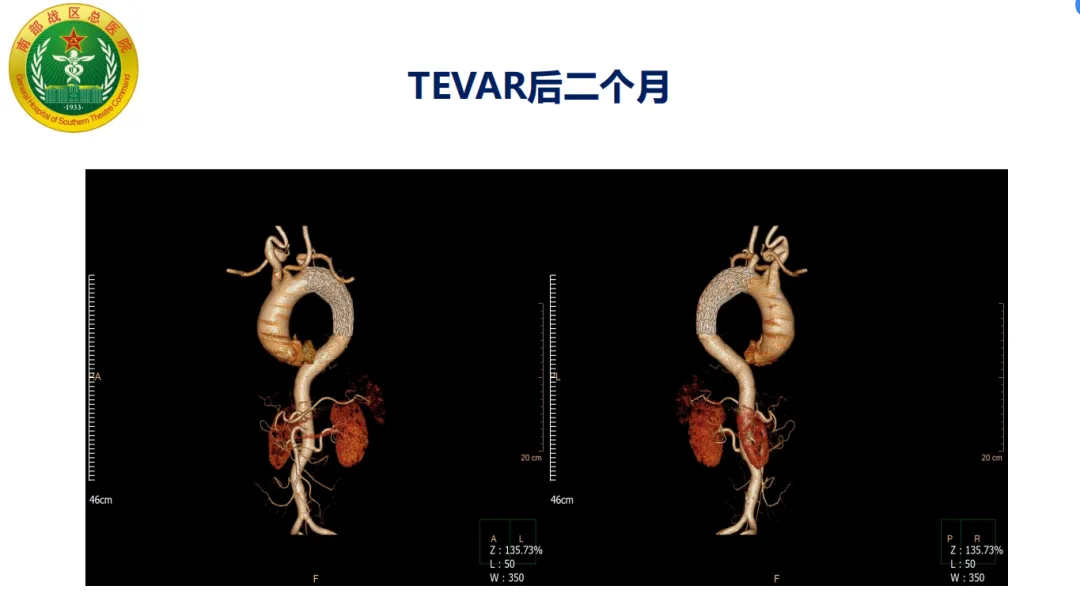
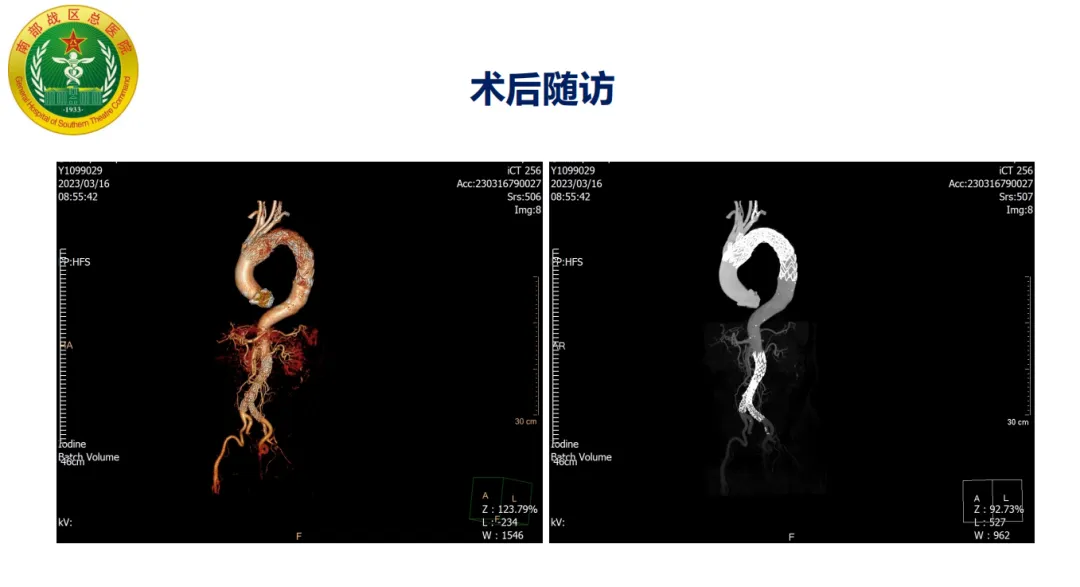
Procedure: The patient had insufficient proximal anchoring zone with hematoma involving the LSA posterior edge. The GORE® TAG® stent system was used with staged release to optimize proximal wall apposition. The proximal part of the stent’s covering was trimmed to expose the bare stent, increasing the anchoring zone and avoiding coverage of the arch branch vessels. Postoperative follow-up showed accurate stent positioning without endoleak.
Case 2:
• Patient: Mr. Liao, 74 years old
• Admission Date: February 13, 2023
• Chief Complaint: Intermittent cough and hemoptysis for over a month
• Past Medical History: Hypertension, BAD-TEVAR (December 2015), AAA-EVAR (April 2022)
• Diagnosis: Proximal retrograde dissection post-BAD endovascular exclusion

Procedure: The new CADS stent was used with staged release, maintaining continuous blood flow throughout the procedure. The stent was released in a controlled manner with precise positioning. The proximal part of the stent’s covering was trimmed to expose the bare stent, increasing the anchoring zone and avoiding coverage of the arch branch vessels. Postoperative follow-up indicated that the procedure was relatively safe, feasible, and effective.
Summary and Outlook
Advantages of the New System: The GORE® TAG® Active Control Thoracic Stent-Graft System, through advancements in materials and engineering, achieves controlled release and precise positioning in complex anatomical structures.
Clinical Outcomes: Studies and clinical cases demonstrate that this system significantly improves the expansion of the proximal anchoring zone, optimizes wall apposition, and effectively reduces complications.

Contact Us
In upcoming articles, we will continue to provide more in-depth analysis of the GORE® TAG® Active Control Thoracic Stent-Graft System. Stay tuned! If you have any questions or interests regarding the GORE® TAG® stent system or the Tianfu Vascular Conference, please leave a comment or contact us via email at endovascluar@simtomax.cn. Thank you for your attention. Let’s work together for health!


