Since the world's first transcatheter aortic valve replacement (TAVR) for aortic stenosis in 2002, TAVR has revolutionized the treatment of aortic stenosis, expanding the indications for TAVR from high-risk populations that cannot tolerate surgical intervention to low and intermediate-risk populations.The treatment of aortic stenosis with TAVR has matured, but the number of cases of aortic regurgitation treated with TAVR in the United States accounts for less than 1% of all TAVR procedures. less than 1% of all TAVR procedures, and many challenges remain in the treatment of aortic regurgitation with TAVR. In recent years, new valve devices have been introduced. In this article, we will discuss the pathophysiology of aortic regurgitation, the timing of treatment, and surgical strategies to treat aortic regurgitation.
Pathophysiologic features of aortic regurgitation
The incidence of aortic regurgitation is about 4.9%, moderate to severe aortic regurgitation is about 0.5%, and the peak incidence is between 40 and 60 years of age. Dilatation of the aortic root combined with congenital bilobed aortic valve malformations, infectious, rheumatic, or degenerative calcific valve disease, and surgical or transcatheter bioprosthetic valve degeneration are the most common causes of aortic regurgitation. The clinical presentation of the patient depends on the severity of the regurgitation; usually, patients with aortic regurgitation are asymptomatic for an extended period of time, even though the definitive diagnosis of aortic regurgitation to the onset of significant symptoms can be as long as 10 to 15 years. In severe cases, the 5-year survival rate after diagnosis and medical treatment is 75%, and the 10-year survival rate is 50%; after the onset of symptoms, the condition deteriorates rapidly, with 50% deaths within 5 years in those with angina pectoris and 50% deaths within 2 years in those with severe left ventricular failure.
From a pathophysiological point of view, severe aortic stenosis is characterized by pressure overload with subsequent centripetal hypertrophy and afterload mismatch. In the vast majority of cases, correction of this mismatch by TAVR surgery results in an increase in the patient's left ventricular ejection fraction and relief of left ventricular hypertrophy. This explains why there is a significant benefit in quality of life and life expectancy in patients with TAVR. The pathophysiology of severe aortic regurgitation, however, is quite different from that of severe aortic stenosis, which is characterized by volume overload and eccentric hypertrophy, i.e., an increase in ventricular volume, virtually no increase in LV wall thickness, and an increase in LV wall stress, followed by progressive LV dysfunction. These structural changes are due to cardiomyocytes being triggered by multiple growth factors that regulate cardiac output through the Frank-Starling mechanism. Once the Frank-Starling mechanism is lost, LV function is irreversibly impaired. Anatomically, degenerative aortic stenosis is the result of progressive calcification of the aortic valve leaflets and annulus, whereas aortic regurgitation is usually the result of leaflet degeneration or insufficiency, aortic root dilatation with enlarged aortic annulus, or both. These anatomic differences present special challenges for TAVR surgery, which we will discuss later.
Timing of treatment for aortic regurgitation
The 2020 ACC/AHA Guidelines for the Management of Valvular Disease recommend the timing of surgical aortic valve replacement (SAVR) for: i) symptomatic severe aortic regurgitation (Class IB recommendation); ii) for asymptomatic severe aortic regurgitation that requires a combined left ventricular ejection fraction (LVEF) ≤55% (Class IB recommendation) or a left ventricular end-systolic diameter (LVESD) >50 mm (Class IIa B Class IB recommendation) or left ventricular end-systolic diameter/body surface area >25 mm/m (Class IIaB recommendation); (iii) Asymptomatic patients with aortic regurgitation who are surgically low-risk, have an LVEF of 55% to 60%, and the past 3 cardiac ultrasounds suggest a progressive decrease in ejection fraction (Class IbB recommendation) or a left ventricular end-diastolic diameter >65 mm (Class IbB recommendation); and (iv) Symptomatic or asymptomatic patients with severe patients with aortic regurgitation requiring surgical bypass grafting, ascending aortic surgery, or other valve surgery (Class IC recommendation).The 2021 ESC/EACTS guidelines for the management of valvular disease also make recommendations for the timing of treatment of aortic regurgitation, which differ slightly from the 2020 ACC/AHA guidelines for valvular disease, as detailed in Table 5.29.
表5.29欧美瓣膜病指南中主动脉瓣反流治疗时机的差异
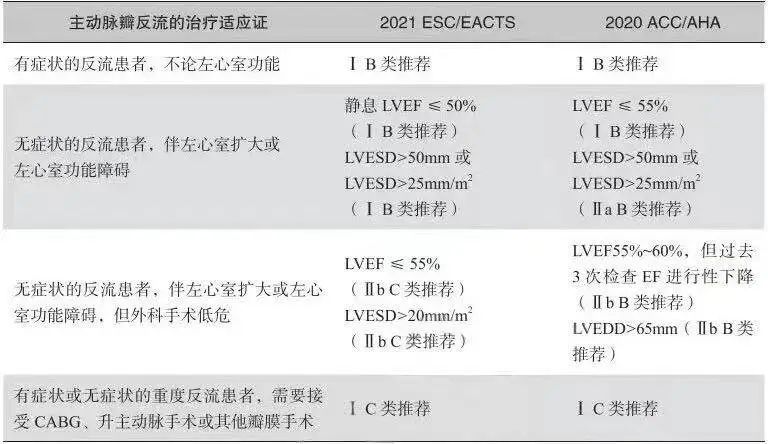
Note:CABG, coronary artery bypass graft; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic diameter; LVEDD, left ventricular end-diastolic diameter.
However, according to data from the European Heart Survey:Only 21.8% and 2.7% of patients with pure aortic regurgitation, LVEF 30% to 50% and less than 30%, respectively, underwent SAVR. Advanced age and associated comorbidities were common reasons for refusal of surgery, and those patients with severe aortic valve closure insufficiency who did not undergo surgical intervention had annual mortality rates of 10% to 20%. In China, the China-DVD study showed that the treatment rate of SAVR in patients with simple regurgitation decreased significantly with age, with a treatment rate of 69.44% in patients aged 60 to 70 years and 37.1% in patients aged 70 to 80 years. Among other things, age and multiple comorbidities are common reasons for limiting surgical open-heart surgery in favor of conservative treatment, thus contributing to the 10% to 20% annual morbidity and mortality rate in this patient population.
Since the risk of surgical intervention increases significantly once cardiac function deteriorates in patients with reflux, can we advance the timing of treatment? Recent studies have shown increased mortality in patients with severe regurgitation despite LVESD >20 mm/m2 and higher LVEF (eg, 60%), suggesting that myocardial dysfunction may occur much earlier than the decline in LVEF or LV enlargement described in current guidelines. LV remodeling due to severe aortic regurgitation is a longitudinal expansion from the base to the apex (similar in shape to an American soccer rather than a basketball), so linear diameters may not reflect progressive changes in LV volume. For example, a patient with severe aortic regurgitation had a transthoracic ultrasound suggesting an LVESD of 40 mm and a left ventricular end-systolic volume (LVESV) of 89 mL 3 years ago, and a follow-up ultrasound suggesting an LVESD of 41 mm 3 years later, but by this time, the LVESV had already gone to 117 mL.In order to more accurately assess regurgitation-induced LV compensation, LV volume, rather than diameter, needs to be continuously Assessment. Recent studies have shown that in patients with aortic regurgitation, LV volume is a better independent predictor of symptoms and death than LV diameter, and a cutoff value of 45 mL/m2 was obtained.More importantly, the correlation between LV diameter and volume was only moderate. Thus, for now, when patients with severe regurgitation present with symptoms, decreased LVEF (LVEF <55%), or significant left ventricular enlargement (LVESD >20 mm/m) that meet current guidelines, there is no question that the patient is an indication for surgical intervention. However, there is also current evidence that when asymptomatic patients with severe regurgitation present with changes in LV volume (LVESV>45mL/m2), patients are at risk for further deterioration of LV function, when surgical intervention may also be justified. In these patients, the combined use of cardiac ultrasound and magnetic resonance provides a more accurate assessment of LV volume changes, pressure load, overall longitudinal strain, and the degree of myocardial fibrosis, metrics that will help the cardiac team decide on the timing of eventual surgery.
Interventional strategies for aortic regurgitation
A significant number of patients with severe aortic regurgitation have contraindications to surgery or are at high risk for only pharmacologic conservative treatment of heart failure due to regurgitation (diuretics, β-blockers, or sarcoplasmic valvuline, etc.), without definitive and effective treatment of the cause of heart failure, i.e., valvular disease. In this context, TAVR has been super-indicated for the treatment of aortic regurgitation in an attempt to reduce mortality and improve quality of life in patients with aortic regurgitation, with varying degrees of success.
01
Technical challenges of interventional therapy
When performing TAVR in patients with aortic regurgitation, the main challenge for the operator is the absence of calcification of the annulus and leaflets, which is essential for valve anchoring and device stability. The absence of calcification, increased output per beat secondary to severe regurgitation, and aortic root dilatation often make prosthetic valve positioning and anchoring very difficult and predispose to valve embolization or displacement, leading to moderate-to-severe perivalvular leakage in the postoperative period. Valve migration can occur within hours of valve implantation, migrate into the aorta causing embolization or penetrate deep into the left ventricle affecting the function of the anterior mitral leaflet. The skirt on the new generation TAVR valve is designed to minimize perivalvular leakage in severe calcific aortic stenosis and may also provide friction for anchoring in patients with aortic regurgitation. In addition, valves with aortic regurgitation are more elastic than those with aortic stenosis and therefore can expand to a greater extent during valve deployment. Standard TAVR sizing calculations may leave the valve significantly undersized.
The risk of valve displacement can be reduced by choosing a larger prosthetic valve (valve oversizing). Current recommendations for oversizing can be 15% to 20%, and oversizing can increase the risk of annular rupture and conduction block. oversizing is calculated using the formula [(circumference or area of the valve anchorage zone/CT circumference or area of the anchorage zone/CT measurement of the annulus)-1]x100%.
Patients with aortic regurgitation usually have aortopathy, associated with congenital anatomical defects or connective tissue disorders characterized by dilatation of the ascending aorta and thin, brittle tissue. In fact, several clinical registry studies suggest that the prevalence of aortopathy in bileaflet aortic valves is 40%. Aortic disease coupled with changes in the valve leaflets and enlargement of the annulus further increases the risk of valve displacement and embolization, leading to worse clinical outcomes with aortic regurgitation-mediated therapy. In patients with suspected thin and brittle aortic wall tissue, it is advisable to use a prosthetic valve device with an adjustable curvature to keep the prosthetic valve coaxial with the aorta to avoid aortic coarctation.
02
Common prosthetic valves for the treatment of regurgitation in China
Currently, prosthetic valves available for TAVR for aortic regurgitation are categorized into specialized and non-specialized valves. Specialized valves include JenaValve and J-Valve, and non-specialized valves are divided into self-expanding valves and ball-expanding valves. Since ball-expanding valves represented by Sapein3 are unsuitable for aortic regurgitation, we mainly focus on self-expanding valves when discussing the treatment of aortic regurgitation by TAVR. Self-expanding valves commonly used in the treatment of aortic regurgitation in China include the EvolutR and the domestically produced Venus-A and VitaFlow valves.Self-expanding valves have been widely used in the treatment of aortic stenosis in the treatment of TAVR, which relies on the anchoring of the annulus and leaflet calcification, whereas specialized valves have been developed for use in cases where there is no calcification, which can be anchored in the annulus and clamped to the aorta, allowing for prosthesis to be used. self leaflets to achieve stabilization of the prosthetic valve device. A selection of classic valves is briefly described below.
The EvolutR (Medtronic) Transcatheter Aortic Valve System is a retrievable, transcatheter implantable aortic valve replacement system in which the prosthetic valve is a self-expanding supra-annular bioprosthetic flap in which the three leaflets and an inner apron (leaflets and inner apron made of a single layer of porcine pericardium) are secured by sutures to a self-expanding, multilayered, radiopaque, nitinol alloy stent, which is made from The Ni-Ti alloy flap holder is laser engraved and cut in one piece, and the surface of the flap holder is electrolytically polished. It is available in four models: 23mm, 26mm, 29mm, and 34mm for flap rings with diameters from 18 to 30mm. It can be repositioned and fully recovered many times even after 80% release; EvolutPRO also has an outer skirt made of porcine pericardial tissue with a height of 1.5 mesh holes on the outer layer of the prosthetic heart valve, which is sutured and wrapped around the inflow portion of the prosthetic heart valve, allowing for a better fixation of the valve in the ventricle and at the level of the annulus where it is horizontally attached to the valve and effectively preventing perivalvular leakage from occurring; EvolutPRO + delivery system has a smaller outer diameter, requiring as little as 5.0 mm for peripheral vessels.
The main design features of VitaFlow are: the valve consists of a self-expanding nickel-titanium alloy stent, three bovine pericardial leaflets, and a double-layered PET skirt. The nickel-titanium alloy stent is designed with a gradual increase in alloy lattice density from the top to the bottom, and the overall morphology is a corolla at the top, with a slightly girdled tube at the middle and lower part of the stent. The outer skirt of the stent covers a height of up to 11mm, ensuring a very good fit to the tissue. It is available in 4 models: 21mm, 24mm, 27mm and 30mm. In addition, the VitaFlow Aortic Valve System's motor-driven delivery system allows for easy handling during implantation, a uniform and controlled release rate, and a stable release position. The second-generation VitaFlow Liberty has a new retractable feature that allows for full retraction of the reset position release after releasing a maximum of 75% of the length, which can improve implantation success in cases with difficult anatomy and in patients with incomplete closure of the aortic valve.
J-ValveTA (Suzhou Jiecheng) is a transapical TAVR system that consists of three U-shaped anchoring rings that grasp the valve leaflets to form a new ring, within which the self-expanding valve is then deployed. It is available in five sizes, 21mm, 23mm, 25mm, 27mm, and 29mm, and is currently CFDA-approved for the treatment of aortic regurgitation and aortic stenosis. J-Valve*TF (Suzhou Jiecheng) is a peripheral transcatheter TAVR system that is currently in clinical studies.
The JenaValve (JenaValve) system consists of a bioprosthetic valve and a self-expanding nickel-titanium alloy stent delivered through the aorta, which features a self-expanding design without a stent mesh that substantially reduces the risk of coronary artery occlusion.The TAVR system is available in sizes of 23mm, 25mm, and 27mm for aortic valve annulus with diameters of 21~27mm. 27mm aortic annulus. The Trilogy Heart Valve System, developed by JenaValve, is the first and only CE-approved transfemoral device of its kind for the treatment of severe symptomatic aortic regurgitation and aortic stenosis. The system also received breakthrough device approval from the U.S. Food and Drug Administration (FDA).The JenaValve Trilogy Valve System consists of a self-expanding nickel-titanium alloy stent and porcine pericardial valve (supra-annular valve design).The design of the transfemoral delivery system utilizes a simple stepped approach to delivery of the bioprosthetic valve in order to allow for anatomical localization within the native valve.The Trilogy Heart Valve System is the first and only device of its kind to receive CE approval for the treatment of severe symptomatic aortic regurgitation and aortic stenosis.
03
Evidence-based medicine for non-specialized valves
Data from previous registries suggest that TAVR-treated patients with aortic regurgitation have a worse prognosis than TAVR-treated patients with aortic stenosis. A study analyzed 331 patients with severe aortic regurgitation treated with TAVR at 40 centers from 2007-2017. Patients had a mean age of 74 years, a mean STS score of 6.7%, 70% had a transfemoral route, and were treated with first- and new-generation ball-expanding and self-expanding valves, with a 30-d all-cause mortality rate, stroke, and vascular complications of 11%, 4%, and 4%, respectively. The rate of remedial valve-in-valve surgery was significantly lower in patients with new-generation valves compared with those with first-generation valves (4% vs. 18%, P<0.01). Another retrospective study analyzed 254 patients with high surgical risk of aortic regurgitation treated with TAVR, with a mean age of 74 years and STS score of 6.6%. The success rate of the newer generation of valve surgery was 82%, with a 9% rate of valve migration and a 4% rate of moderate-to-severe postoperative regurgitation. Both undersized and oversized valves were associated with an increased risk of valve migration. Despite better outcomes with new-generation valves than with first-generation valves, patients with aortic regurgitation continue to have inferior outcomes to patients with aortic stenosis treated with TAVR.
An analysis of a TAVR registry that included patients with aortic regurgitation with degraded bioprosthetic valve function showed similar results:A total of 146 patients were included in the analysis, of whom 78 had their own aortic regurgitation and 68 had degraded aortic bioprosthetic valves. The success rate of new-generation valve surgery was 85%. The most common reasons for surgical failure were the need for a second transcatheter valve implantation and the development of moderate-to-severe perivalvular regurgitation, with an incidence of 3%. A recent meta-analysis evaluating the prognosis of patients with aortic regurgitation treated with TAVR, which included 11 clinical studies, showed that among 911 patients with aortic regurgitation treated with TAVR, the device success rate was 80%, the incidence of moderate or greater perivalvular leakage was 7%, the incidence of vascular complications was 6%, and the 30-d and 1-year mortality rates were 10% and 19%. Compared with the first-generation valves, the new-generation valves had a lower percentage of valve-in-valve (22% vs. 5%, P<0.001) and a lower incidence of moderate-to-severe perivalvular leaks (17% vs. 3%, P<0.001).
Despite the promise of TAVR for the treatment of aortic regurgitation, the use of self-expanding valves for TAVR in clinical practice is considered to be indicated only in high-risk aortic regurgitation patients who do not qualify for surgical intervention. Therefore, there is a need to design valves specifically for simple aortic regurgitation.
04
Evidence-based medicine for specialized valves
New valves have been developed specifically for the treatment of aortic regurgitation, and the JenaValve is one such valve that uses a nickel-titanium alloy ring around the aortic valve leaflets as an anchor point within which the valve can deploy. An early German multicenter registry study enrolled 31 patients with transapical implantation of the JenaValve for aortic regurgitation, with a mean age of 74 years, a EuroScore of 24%, and 30 (97% of patients were successfully implanted with the JenaValve.) The all-cause mortality rates at 30 d and 6 months were 13% and 19%, respectively, with transapical access being a significant risk factor for death. The JenaValve Trilogy Heart Valve System is the world's first and currently only CE-approved transfemoral TAVR system for the treatment of severe symptomatic aortic regurgitation or aortic stenosis. In the 30-d follow-up data of 70 cases from the ALIGN-AR study published in TVT 2021, the all-cause mortality rate was only 2.9%, the cardiac mortality rate was 1.4%, there were no disabling strokes, the surgical success rate for single valve implantation was 95.7%, there was only 1 case of more than moderate perivalvular leak, and the permanent pacemaker rate was 22.9%. In addition, it has been recently reported in the literature that intraoperative transesophageal ultrasound allows for commissural alignment of the trans-femoral JenaValve Trilogy valve to the autograft leaflets because of its 3 locators.The ALIGN-AR PIVOTAL study (NCT04415047) is the most important of the current JenaValve studies. currently the most important study of JenaValve and will evaluate the safety and efficacy of the trans-femoral JenaValve Trilogy Heart Valve System in the treatment of patients with symptomatic, surgical high-risk severe aortic regurgitation. The study, which is currently in the enrollment phase, proposes to implant the JenaValve in 180 patients with symptomatic, surgically high-risk severe aortic regurgitation, with the primary endpoint of the study being all-cause mortality at 1 year. If positive results are achieved in this study, the U.S. FDA will approve its indication for aortic regurgitation.
The domestically produced J-valve has some design similarities to the JenaValve, but the removable connection between the J-Valve support structure and the fixator allows for more flexibility in adjusting the position of the valve. Multicenter studies in small samples have demonstrated its safety and feasibility. The largest-sample single-center study in cardiac surgery at West China Hospital of Sichuan University enrolled 134 patients with aortic regurgitation, all of whom successfully underwent TAVR, except for 5 patients who were ultimately converted to surgical valve replacement due to coronary artery obstruction, valvular thrombus, valvular displacement, and moderate perivalvular leakage, respectively. 74.5% of the patients included in the study were male, with a mean age of 73.1 years, a mean EuroScore II score of 11.5% ± 6.8%, and a mean STS score of 9.8% ± 5.3%. At 30-d postoperative follow-up, a total of 4 patients (3.0%) died; 12 patients developed third-degree atrioventricular block requiring permanent pacemaker implantation; and device implantation was successful in 96.3% of cases. At 6-month postoperative follow-up, there were 5 cumulative deaths (3.7%). A new generation of J-Valve with transfemoral access has been developed, and clinical trials on product safety and efficacy are underway.
The meta-analysis suggested that the surgical success rate of overall TAVR in patients with aortic regurgitation was 89.9% (95% confidence interval: 81.1%~96.1%), and the risk of perioperative hemorrhage was 6.4% (95% confidence interval: 2.9%~10.8%). The all-cause mortality rate in the 30d postoperative period was 10.4% (95% confidence interval: 7.1%-14.2%), the incidence of stroke was 2.2% (95% confidence interval: 0.9%-3.9%), and 10.7% of the patients required implantation of a permanent pacemaker (95% confidence interval: 7.3%-14.6%). The risk of moderate-to-severe aortic regurgitation at 30 d after TAVR was 11.5% (95% confidence interval: 2.9% to 23.6%). In the dedicated valve subgroup, the TAVR surgical success rate was 93.0% (95% confidence interval: 85.9% to 98.1%), the all-cause mortality rate at 30 d postoperatively was 9.1% (95% confidence interval: 3.7% to 16.0%), and more than trace aortic regurgitation was present in 2.8% (95% confidence interval: 0.1% to 7.6%) of patients. Compared with first-generation valves, newer-generation valves for aortic regurgitation had a significantly lower 30-d all-cause mortality rate (7.1% vs. 15.6%, P=0.02) and a significantly higher surgical success rate (92.9% vs. 68.4%, P<0.001). Compared with other new-generation, non-dedicated valves, the dedicated valves JenaValve and J-Valve did not have a reduction in all-cause mortality (9.1%v.5.9%, P=0.50) or an improved risk of aortic regurgitation above the trace level (2.8%vs.4.4%, P=0.65) but had an improved surgical success rate (93.0%vs.83.6%, P =0.042).
05
Surgical strategies for interventional therapy
The main challenges of transfemoral TAVR for simple aortic regurgitation include:1) aortic valve without calcified thickening, difficulty in anchoring existing prosthetic valves, and high probability of valve prolapse and valve-in-valve. ② With the progression of aortic valve regurgitation, patients with poor cardiac function are prone to circulatory collapse and malignant arrhythmias. ③ Compared with patients with aortic stenosis, the rate of postoperative left bundle branch block and pacemaker implantation is higher. Therefore, transfemoral TAVR for pure aortic regurgitation should be performed with strict patient screening and in experienced and mature centers.
1. Careful preoperative analysis of CT to select patients with anatomically appropriate regurgitation
(1) The annulus is generally large in patients with simple regurgitation, and the maximum bottom edge diameter of the currently marketed self-expanding valves in China is 32 mm. Considering the implanted valve Oversizing rate of 15%-20%, TAVR is recommended for the treatment of patients with aortic regurgitation with an annulus diameter of <27 mm (for reference only, individualized formulation of strategies).
(2) A suitable LV outflow tract and annulus structure, which is the first anchoring point of valve contact, is available. Ideally, the diameter of the outflow tract should not differ significantly from the diameter of the annulus (straight), and the outflow tract should have a certain length (measured 4 mm below the annulus).
(3) If the valve leaflets are characterized by thickening, adhesions, calcification, or fusion of the valve junction, this will increase the anchoring force of the valve to a greater extent.
(4) The use of sinotubular junction or ascending aorta anchored to the valve corolla improves the stability of the valve after implantation. Different sizes of valve corolla diameters are different, the more appropriate is: the diameter of the ascending aorta 40mm height is smaller than the diameter of the chosen valve corolla.
2.TAVR intraoperative techniques
(1) It is recommended to operate under general anesthesia, and should ensure that the patient is in a sufficiently sedated state during the valve release to prevent the valve release from being affected by the patient's agitation.
(2) Transesophageal echocardiography can be used to assist in intraoperative valve localization and to accurately assess the degree of postoperative valve regurgitation. Dual-modality imaging with transesophageal ultrasound and DSA is recommended to guide the procedure.(3) Because the aortic annulus is the primary anchoring site for the valve, the most supportive portion of the valve should be placed at the level of the annulus. Due to the lack of anchoring on the valve, the valve should be implanted 2 to 3 mm lower than in patients with aortic stenosis in order to prevent valve prolapse.
(4) During valve release, ensure that temporary pacing is stable, and improve the stability of valve release by rapid pacing or even by stopping breathing through the anesthesia machine.
(5) If a valve-in-valve is required, it is recommended that the second valve be assisted to pass through the initial valve by utilizing a circler to prevent the second valve from dislocating the initial valve as it passes through.
(6) For patients with markedly enlarged hearts and markedly reduced cardiac function, a backup circulatory assist device is recommended to cope with a variety of unforeseen events.
3. Precautions after TAVR surgery
(1) Due to the lack of adequate anchoring in patients with simple regurgitation, there is still a possibility of valve displacement after surgery, and dynamic review of transthoracic ultrasound is needed in the monitoring ward after surgery.
(2) Prosthetic valve position tends to be deeper in patients with regurgitation, and the risk of complete left bundle branch block or even third-degree atrioventricular block is higher in the postoperative period than in patients with aortic stenosis. If necessary, a temporary pacemaker should be in place to prolong the duration of the monitoring period (individualized assessment).
(3) Left bundle branch pacing is recommended if severe bradyarrhythmias are present and permanent pacemaker implantation is indicated.
06
SEASON-AR researches
Due to left ventricular dilatation and left ventricular insufficiency, patients with aortic regurgitation who are candidates for TAVR tend to have worse clinical outcomes than many patients with aortic stenosis. Perioperative technical difficulties and the lack of a dedicated transfemoral valve system have made TAVR an “off-label” option for aortic regurgitation. Although new-generation valves have achieved good results in aortic regurgitation, with better endpoints than first-generation valves in terms of valve displacement, valve-in-valve, and moderate-to-severe postoperative regurgitation, their clinical outcomes are far from those of TAVR for aortic stenosis. To date, there is still a lack of randomized controlled studies to demonstrate the efficacy of TAVR in the treatment of aortic regurgitation, and there is no standard procedure for TAVR in the treatment of aortic regurgitation. Professor Chen Shaoliang and Professor Zhang Junjie of Nanjing First Hospital initiated the “Transcatheter Self-Expanding Valve Implantation for the Treatment of Severe Aortic Valve Closure Insufficiency: A Multicenter, Prospective, Randomized Controlled Study (SEASON-AR Study, ClinicalTrials.gov Identifier: NCT04864145)”, which aimed to evaluate the efficacy of TAVR for the treatment of aortic regurgitation in a multicenter, prospective, randomized controlled study. “, which was designed to compare the safety and efficacy of TAVR therapy with pharmacologic conservative therapy in 210 patients with severe aortic regurgitation who were not surgical candidates.
1.Inclusion criteria for the study
(1) Severe aortic regurgitation with a mean transvalvular pressure difference of less than 20 mmHg.
(2)Indications for surgical intervention: symptomatic severe regurgitation; asymptomatic but combined LVEF ≤55% or LVEDD>65mm or LVESD>50mm.
(3)Measurement of annular circumference ≤85mm by MDCT or 3D-TEE.
(4)MDCT or 3D-TEE showed that the ratio of the 4-mm circumference of the LV outflow tract to the circumference of the annulus was 0.95-1.05.(5) STS score ≥8 or moderate-to-severe weakness or refusal of surgical valve replacement, or the presence of any of the following risk factors judged to be difficult to perform surgical aortic valve surgery: (i) porcelain aorta or active aortic atheroma of the ascending aorta; (ii) mediastinal treatment with Radiotherapy to the mediastinum; (3) previous mediastinitis; (4) presence of patent coronary artery bypass grafts; (5) more than two cardiothoracic surgeries; (6) cirrhosis of the liver; and (7) other surgical risk factors.
2. Exclusion criteria for the study (1) Age <60 years.
(1) Age <60 years.
(2)Diameter of ascending aorta >45mm.
(3) Multiple coronary artery lesions (SYNTAX score >32).
(4)Life expectancy <1 year.
(5)LVEF <30%.
(6)Acute myocardial infarction within 30d.
(7) Allergy or contraindication to relevant medications (aspirin, clopidogrel, warfarin, or contrast agents). (8) Other conditions judged by the investigator to make participation in the study unsuitable.
The primary endpoint of the study was the composite endpoint of:all-cause death, disabling stroke, or rehospitalization for heart failure at 12 months postoperatively.
Summary and outlook
A significant number of patients with severe aortic regurgitation are at unacceptable surgical risk, and early intervention in asymptomatic patients with severe regurgitation may be justified, with numerous imaging studies now devoted to finding the right time for surgical intervention. And current clinical work is needed to treat many patients with severe regurgitation who are at high risk for surgical intervention and who may benefit from TAVR. The new generation of TAVR valves currently used for aortic stenosis has demonstrated safety and feasibility for use in patients with aortic regurgitation. However, registry data show a poorer overall prognosis in this group of patients. These results may be due to the inability of noncalcified valves to provide an anchoring zone for the TAVR device. This lack of stability leads to an increased incidence of valve displacement and perivalvular leakage, with subsequent increased postoperative moderate-to-severe regurgitation and mortality. New TAVR valves specifically for aortic regurgitation are in development, and early studies have shown favorable results. Randomized controlled studies and research on new methods of valve anchoring are still needed to ultimately make TAVR available for the treatment of aortic regurgitation.
Bibliography
[1]MORI M, GUPTA A, WANG Y, et al. Trends in Transcatheter and Surgical Aortic Valve Replacement Among Older
Adults in the United States[J]. JAm Coll Cardiol, 2021,78(22):2161-2172.
[2]BHOGALS, ROGERS T, ALADIN A, et al. TAVR in 2023: Who Should Not Get It [J]. Am J Cardiol, 2023,193:1-18.[3]MARKHAM R, GHODSIAN M, SHARMAR. TAVRin Patients with Pure Aortic Regurgitation: Ready to Use [J]. Curr
Cardiol Rep, 2020,22(9):98
[4] ARIAS EA, BHAN A, LIM ZY, et al. TAVI for Pure Native Aortic Regurgitation: Are We There Yet [J]. IntervCardiol,
2019,14(1):26-30.
[5] WRITING COMMITTEE M, OTTO CM, NISHIMURA RA, et al. 2020ACC/AHA Guideline for the Management of
Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines[J]. JAm Coll Cardiol, 2021, 77(4):e25-e197.
[6] VAHANIAN A, BEYERSDORFF, PRAZ F, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart
disease[J]. Eur Heart J, 2022,43(7):561-632.
[7] IUNG B, BARON G, BUTCHART EG, et al. A prospective survey of patients with valvular heart disease in Europe: The
Euro Heart Survey on Valvular Heart Disease[J]. Eur Heart J, 2003,24(13):1231-43.
[8]RAHHAB Z, EL FAQUIR N, TCHETCHE D, et al. Expanding the indications for transcatheter aortic valve
implantation[J]. Nat Rev Cardiol, 2020, 17(2):75-84.
[9] XU H, LIU Q, CAOK, et al.Distribution,Characteristics, Management of Older Patients With Valvular Heart Discase in
China[J].JACC: Asia,2022.
[10] YANG LT, MICHELENA HI, SCOTT CG, et al. Outcomes inChronic Hemodynamically Significant Aortic
Regurgitation and Limitations of Current Guidelines[J]. JAm Coll Cardiol,2019,73(14):1741-1752.
[11] ANAND V, NISHIMURA RA, RIGOLIN VH. Earlier Intervention in Asymptomatic Chronic Aortic Regurgitation-
Novel Indicators of Myocardial Overload[J]. JAMA Cardiol, 2022,7(9):883-884.
[12]ANAND V, YANG L, LUIS SA, et al. Association of Left Ventricular Volume in Predicting Clinical Outcomes in Patients with Aortic Regurgitation[J]. JAm Soc Echocardiogr, 2021, 34(4):352-359.
[13] YANG LT, ANAND V, ZAMBITO EI, et al. Association of Echocardiographic Left Ventricular End-Systolic Volume
and Volume-Derived Ejection Fraction With Outcome in Asymptomatic Chronic Aortic Regurgitation[J]. JAMA Cardiol,2021,6(2):189-198.
[14] MALAHFJI M, CRUDO V, KAOLAWANICHY, et al. Association of Cardiac Remodeling with Aortic Regurgitation
Outcomes: The AR Consortium of theSCMR Registry[J]. J Am Coll Cardiol, 2023.
[15] HASHIMOTO G, ENRIQUEZ-SARANO M, STANBERRY LI, et al. Association of Left Ventricular Remodeling
Assessment by Cardiac Magnetic Resonance With Outcomes in Patients With Chronic Aortic Regurgitation[J]. JAMA Cardiol, 2022, 7(9):924-933.
[16]KOCKOVA R, LINKOVA H, HLUBOCKA Z, et al. Multiparametric Strategy to Predict Early Discase Decompensation
in Asymptomatic Severe Aortic Regurgitation[J].Cire Cardiovase Imaging, 2022, 15(12):e014901.
[17] DVIR D, WEBB JG, PIAZZA N, et al. Multicenter evaluation of transcatheter aortic valve replacement using either
SAPIEN XT or CoreValve: Degree of device oversizing by computed-tomography and clinical outcomes[J]. Catheter Cardiovasc Interv, 2015,86(3):508-515.
[18] ARORA S, LAHEWALA S, ZUZEK Z, et al. Transcatheter aortic valve replacement in aortic regurgitation: The U.S.
experience[JJ. Catheter Cardiovasc Interv, 2020.
[19] YOON SH, SCHMIDT T, BLEIZIFFER S, et al. Transcatheter Aortic Valve Replacement in Pure Native Aortic Valve
Regurgitation[J]. J Am Coll Cardiol,2017,70(22):2752-2763.
[20] DE BACKER O, PILGRIM T, SIMONATO M, et al. Usefulness of Transcatheter Aortic Valve Implantation for
Treatment of Pure Native Aortic Valve Regurgitation[J]. Am JCardiol, 2018, 122(6):1028-1035.
[21] FRANZONE A, PICCOLO R, SIONTIS GC, et al. Transcatheter Aortic Valve Replacement for the Treatment of Pure
Native Aortic Valve Regurgitation: A Systematic Review[J].JACC Cardiovase Interv, 2016,9(22):2308-2317.
[22 ] HIRA RS, VEMULAPALLI S, LI Z, et al. Trends and Outcomes of Off-label Use of Transcatheter Aortic Valve
Replacement: Insights From the NCDR STS/ACC TVT Registry[J]. JAMA Cardiol, 2017,2(8):846-854.
[23 ] SAWAYA FJ, DEUTSCH MA, SEIFFERT M, ct al. Safety and Efficacy of Transcatheter Aortic Valve Replacement
in the Treatment of Pure Aortic Regurgitation in Native Valves and Failing Surgical Bioprostheses: Results From an International Registry Study[J].JACC Cardiovasc Interv,2017,10(10):1048-1056.
[24] TAKAGI H, HARI Y, KAWAI N, et al. Meta-Analysis and Meta-Regression of Transcatheter Aortic Valve Implantation
for Pure Native Aortic Regurgitation[J]. Heart Lung Cire, 2020,29(5):729-741.
[25] SEIFFERT M, BADER R, KAPPERT U. et al. Initial German experience with transapical implantation of a second-
generation transcatheter heart valve for the treatment of aortic regurgitation[J]. JACC Cardiovasc Interv, 2014,7(10):1168-1174.
[26] HAMID N, RANARD LS, KHALIQUE OK, et al, Commissural Alignment After Transfemoral Transcatheter Aortic
Valve Replacement With the JenaValve Trilogy System[J]. JACC Cardiovasc Interv,2021,14(18):2079-2081.
[27] LIU L, CHENS, SHI J, et al. Transcatheter Aortic Valve Replacement in Aortic Regurgitation[J]. Ann Thorac Surg,
2020,110(6):1959-1965.
[28] WERNLY B, EDER S, NAVARESE EP, et al. Transcatheter aortic valve replacement for pure aortic valve regurgitation:
“on-label”versus “off-label”use of TAVR devices[JJ. Clin Res Cardiol, 2019,108(8):921-930.
The book is edited by Prof. Wu Yongjian and Prof. Wang Jiguang, reviewed by Prof. Chen Shaoliang and Prof. Yuan Zuyi, with Prof. Gao Zhan, Prof. Song Guangyuan, and Prof. Huang Qifang as the academic secretaries, and more than 200 experts and scholars.


