Preamble
Clinical practice guidelines summarize and evaluate all the relevant evidence on a particular topic at the time of their development, with the goal of helping physicians choose the best management strategy for an individual patient in a given situation. These guidelines consider the impact of different diagnostic or therapeutic approaches on a patient's prognosis and the risk-benefit ratio. Although these guidelines are not a substitute for textbooks, they supplement them and cover topics relevant to contemporary clinical practice. They are an important tool to help physicians make decisions in their daily practice. In essence, however, while these recommendations are a valuable resource for guiding clinical practice, their application should always be tailored to the needs of the individual patient. Each patient's situation is unique, presenting its own set of variables and circumstances. The guidelines are a tool designed to support, not replace, the physician's decision-making process based on his or her knowledge, expertise, and understanding of the individual patient's situation. Furthermore, the guidelines should not be interpreted as a legally binding document. The legal responsibilities of healthcare professionals remain firmly grounded in applicable laws and regulations, and these guidelines do not alter those obligations.
Pathophysiology and natural disease processes:
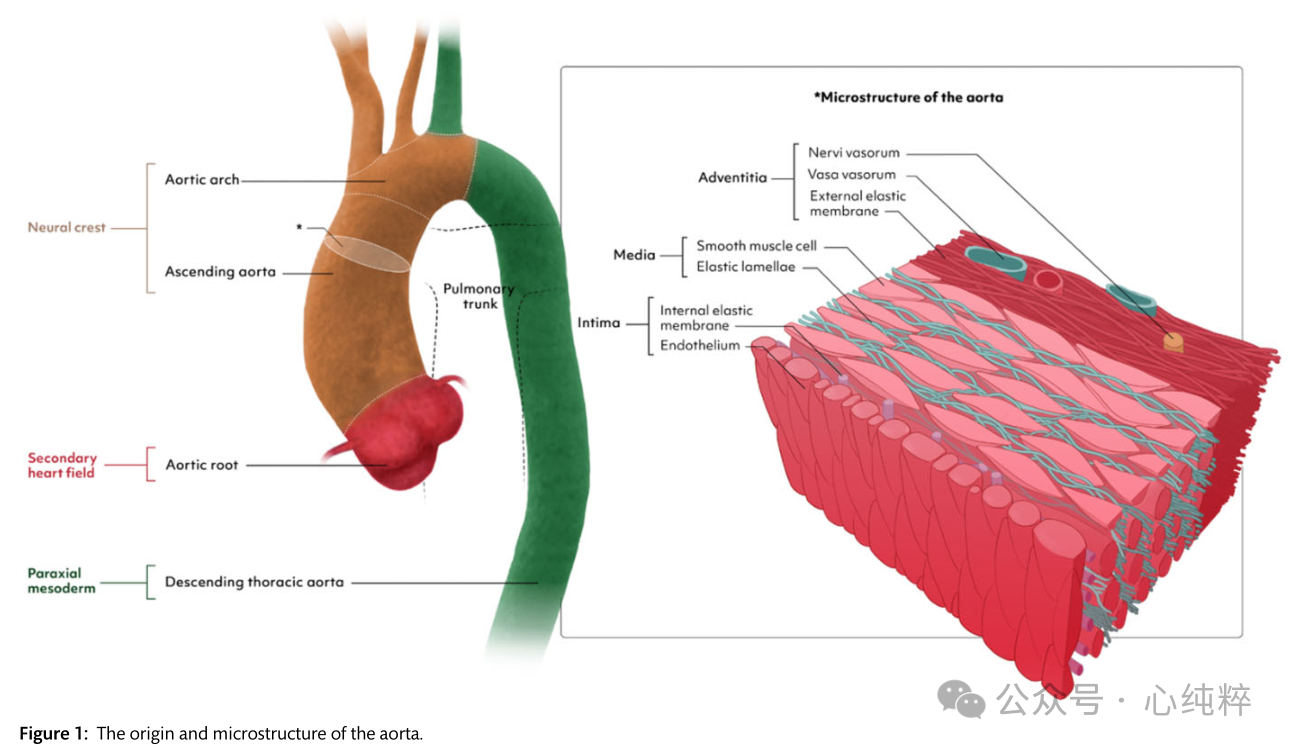
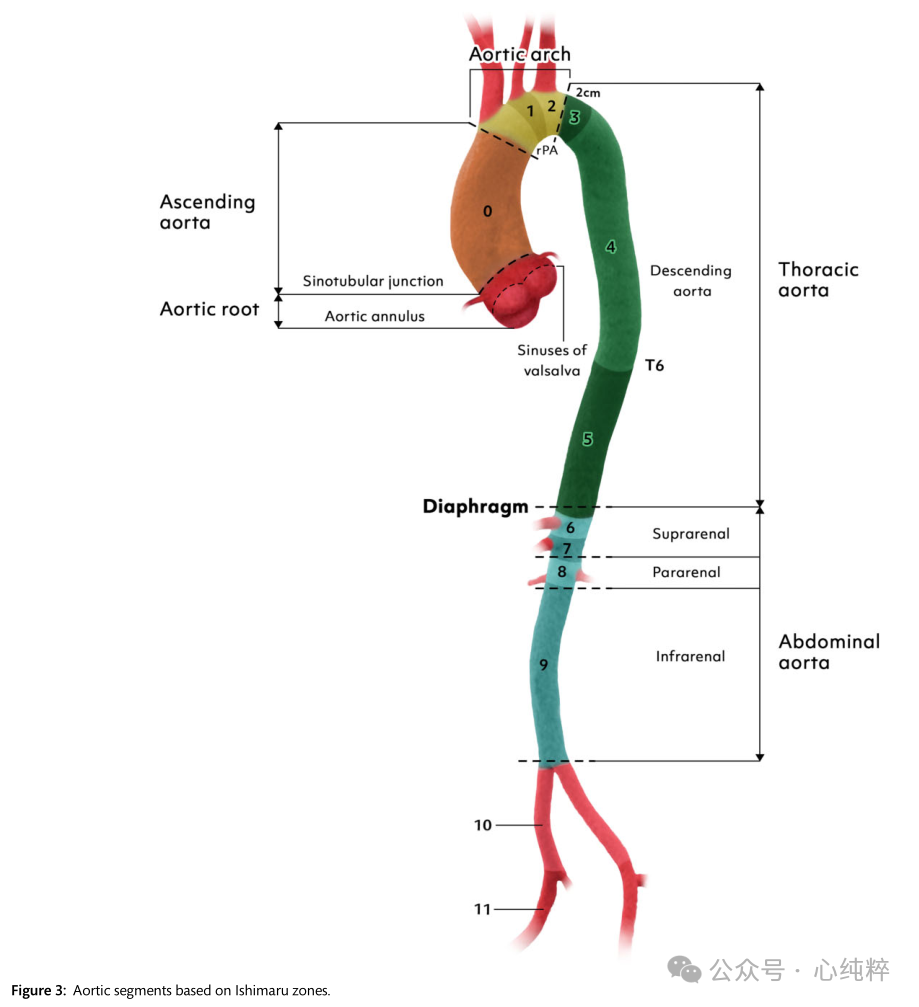
The origin and microstructure of the aorta is shown in Figure 1. Initially, a pair of aortas was formed by the fusion of isolated vascular islands. The aortic bursa is formed by the fusion of the two abdominal and descending aortas and the dorsal aorta. Six paired aortic arches develop in succession, connecting the aortic sac to the dorsal aorta. These arches develop and recede at different times, resulting in the formation of the aortic arch, supra-aortic branches, pulmonary arteries, and ductus arteriosus. Most congenital malformations of the great vessels are due to the persistence of vascular segments in normal conditions and vice versa.
Vascular smooth muscle cells are the major cell population in aortic tissue and interact with the extracellular matrix (ECM) to maintain aortic homeostasis.Different models of AD describe changes leading to loss of contractile function of smooth muscle cells and phenotypic switching to similar cells of different origins (e.g., mesenchymal cells or myofibroblasts). In the context of AD, the expression of smooth muscle cell proteins is reduced and the expression of inflammatory proteins such as matrix metalloproteinases is increased.
Fibronectin 1 is an important component of the ECM and plays a key role in the transmission of mechanical stress between the ECM and vascular smooth muscle cells. Current data suggest that the interaction between vascular smooth muscle cells and mechanical stimuli in the ECM is a key factor in the development of AD. Aging and Alzheimer's disease typically result in a loss of dilatation, which in turn is associated with an increase in glycosaminoglycans and extracellular collagen and a decrease in elastin content. Genetic variants associated with the transforming growth factor-β (TGFβ) signaling pathway have been linked to aneurysm formation in the context of inherited connective tissue disorders, of which Marfan's and Loeys-Dietz syndromes are the most prominent.TGFβ is a cytokine ubiquitously present in most mammalian cells, controlling proliferation and cellular differentiation.Antibodies to TGFβ in the Marfan's syndrome mouse model prevented aneurysm formation in the Marfan syndrome mouse model, thus establishing a causal link to AD development at the molecular level. In the past decade, several TGFβ-associated vascular lesions have been identified and the TGFβ signaling pathway has been transformed into a promising target for therapeutic intervention.
Epidemiology: Epidemiologic studies of the thoracic aorta in populations are scarce because such studies require adequate serial imaging [at least transthoracic echocardiography (TTE) and preferably computed tomography (CT) or magnetic resonance angiography (MR angiography)] of a large number of individuals in a specific geographic area. Descending and abdominal aortic aneurysms were defined as 1.5 times the diameter of a normal vessel, and aortic root and ascending aorta were defined as >45 mm. aortic root and ascending aortic dilatation were defined as a diameter of between 40 and 45 mm.
In a study conducted in Canada, the incidence of thoracic aortic aneurysm (TAA) increased from 3.5 to 7.6 cases per 100,000 people between 2002 and 2014. In a prospective cohort study in Sweden, the risk incidence per 100,000 patient-years was 15 cases of aortic coarctation, 27 cases of abdominal aortic aneurysm, and 9 cases of thoracic aortic aneurysm. In a review of 11 studies, the incidence of thoracic aortic aneurysms ranged from 5 to 10 per 100,000 person-years. In a recent study, enlarged ascending aorta (>36 mm) was found in 12% of participants in Iran, while the prevalence of aneurysms (>45 mm) was 1.2%. All of these data need to be read with caution because ultrasound measurement of aortic size varies (outside-out, inside-in, or edge-to-edge methods). The results of a recent study suggest that aortic normal values may be different in Africans and that race-specific normal values are needed.
Epidemiologic information on acute aortic syndromes (AASs) is limited. In autopsy studies, the prevalence of aortic coarctation ranged from 0.2% to 0.8%, while in most populations the incidence of aortic coarctation ranged from 0.61 to 7.2 per 100,000 population. In a study conducted in the United States during 2010-2015, the prevalence rates of coarctation, intramural hematoma (IMH), and penetrating aortic ulcer (PAU) were 4.4, 1.2, and 2.1 per 100,000 person-years, respectively.However, these rates appear to underestimate the actual prevalence rates, as many victims die without receiving a diagnosis or before they reach the hospital. A Japanese study reported a much higher incidence rate (17.6/100,000), which may be more accurate because the authors performed systematic CT scans of all out-of-hospital decedents without a clear cause of death and reported 30-day mortality rates ranging from 11% to nearly 75%, the latter of which was reported in Japan using a postmortem imaging methodology, and in a more recent analysis in Spain and Ontario over the past 20 years, the rate of diagnosis of aortic coarctation was followed by an increase in surgical rates and a decrease in mortality.
Epidemiologic information on abdominal aortic aneurysms (AAA) is less available, not only because of the ease of diagnosis using abdominal ultrasound, but also because of some national population screening studies. Overall, the prevalence of AAA is declining:Whereas in population studies and screening trials in the late 20th century, the prevalence in men was about 4% - 5%, more recent studies have reported even lower rates and are often used to refute national screening programs. In a Danish vascular screening trial, 3.3% of participants had AAA. in a Swedish national AAA screening program for men over 65 years of age, the prevalence of AAA was 1.5%. Reduced smoking prevalence and improved management of cardiovascular risk factors may plausibly explain this evolution. As a result, a number of countries have reported declines in hospitalization and mortality rates associated with AAA.
Acute aortic coarctation:
The location of the main entrance tear and the extent of aortic wall stripping (e.g., the process of detachment) determine the pathologic-anatomic classification and essentially the treatment strategy.
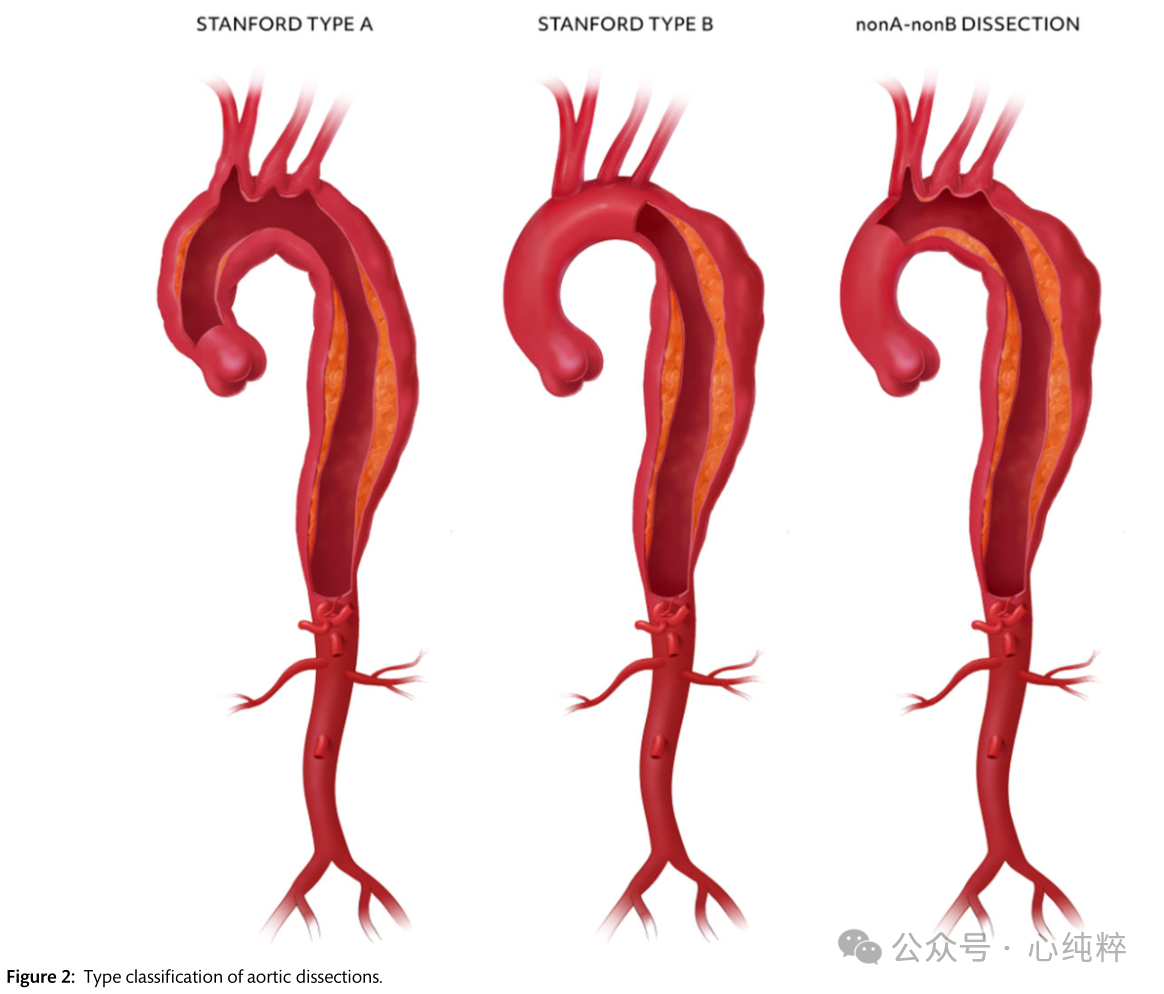
Proximal or type A aortic coarctation involves the ascending aorta and requires open surgery in almost every patient. Type B coarctation, on the other hand, does not include the ascending aorta and can be medically treated in many patients in conjunction with endovascular intervention. Improving Diagnostic and Imaging Quality A subgroup of nona-nonb clips has recently been identified that includes separation of the mesenteric layer around the aortic arch but excludes the ascending aorta, which typically presents with an access tear in the region between the cephalic brachial artery and the left subclavian artery. These features should be clearly distinguished from the earlier non-type A non-type B entrapment, which is defined by an entry tear and retrograde extension of the arch and is nothing more than an overlap and subset of type A entrapment.
Proximal or type A aortic coarctation accounts for 59-67% of all cases of acute aortic syndrome, type B coarctation for about 31%, and non-a non-B coarctation for 3-10%, with a few cases showing an entry tear located not in the arch region but in the descending aorta, with retrograde dilatation of the pseudolumen.
With the recent interest in non-a non-b dissection, the view of optimal treatment is ambiguous, with options ranging from open surgical resection of the arch (with or without elephant trunk) and hybrid debranching and/or endovascular interventions to medical therapy only. Aortic coarctation is further subdivided on the basis of symptoms/coarctation episodes: (i) acute (within 14 days of coarctation episode); (ii) subacute (15-90 days after coarctation onset) and (iii) chronic (91 days after coarctation onset and beyond).
Acute type A aortic dissection (ATAAD) is a serious surgical emergency requiring immediate intervention in most cases. Despite significant advances in surgical technique and perioperative management, repair of type A clamps continues to be associated with substantial mortality and morbidity, with a reported mortality rate of more than 20%, depending on current characteristics and surgical experience.Survival of patients with repaired type A clamps is largely dependent on their hemodynamic status and the presence of intra-organ ischemia.
Although the majority of patients with acute type A clamps will benefit from immediate surgery, the development of clamp-specific risk models capable of rapid risk assessment at the bedside has provided clinicians with additional tools to optimize their practice. These models may pave the way for advanced decision making in situations where surgical risk is particularly high, potentially favoring endovascular treatment to address poor perfusion followed by open aortic repair or even endovascular primary repair in the future. Risk stratification also provides a simple and reproducible method for assessing risk-adjusted outcomes for quality improvement, performance measurement, or comparative effectiveness assessment of different techniques. Bedside tools commonly used for risk assessment in cardiac surgery, such as the STS score and EuroSCORE II, are not calibrated for use in assessing risk in patients with ATAAD.
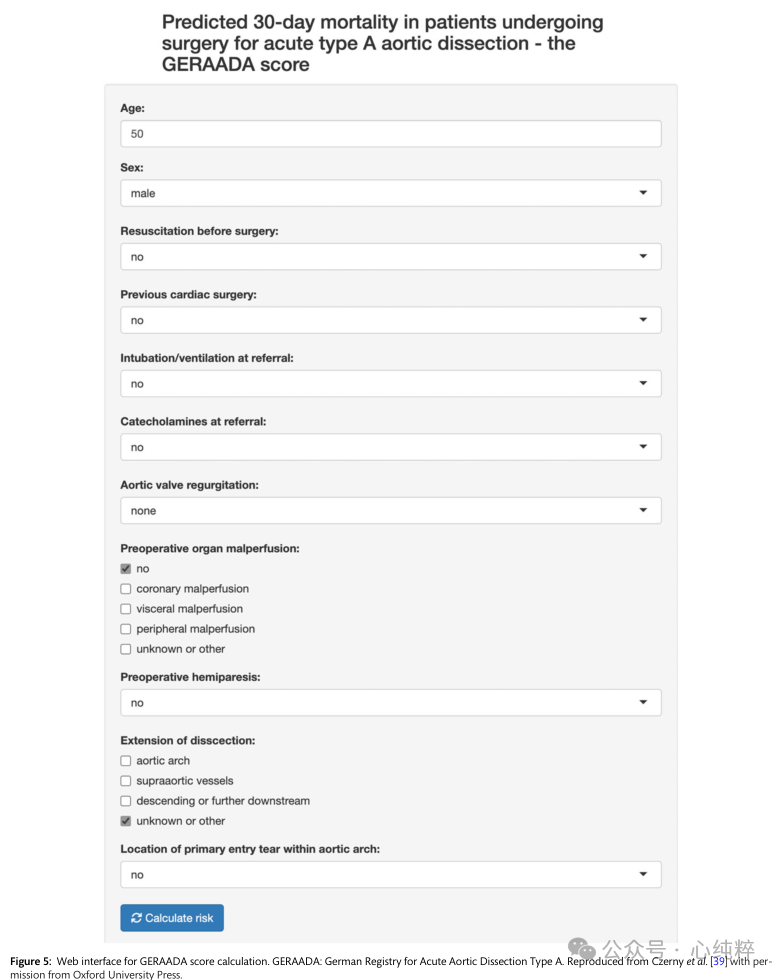
Two risk assessment methods are currently used for type A coarctation:the German Registry for Acute Aortic Coarctation Type A (GERAADA) score and the Penn classification.The GERAADA score is calculated using a web-based application and categorizes patients into low-risk (<15%), intermediate-risk (15-30%), and high-risk (>30%) mortality groups (https://www.dgthg .de/de/ GERAADA_Score).Inputs to the GERAADA score include gender, age, preoperative resuscitation status, previous cardiac surgery, intubation, catecholamine support at the time of referral, grade of aortic regurgitation, organ malperfusion, hemiparesis, extension of aortic coarctation, and location of primary tear.The GERAADA score is an effective predictor of 30-day mortality, and has been validated in various cohorts, although it may overestimate overall risk in some populations.
The Penn classification is a very concise patient classification based on systemic ischemic burden that ranges from none (Penn class a) to localized or regional ischemia (Penn class B), global ischemia or shock (Penn class C), or localized and global ischemia (Penn class BC). The system has been validated to emphasize the burden of total physiologic ischemia and to account for hemodynamic failure in the setting of localized ischemia.The baseline mortality rate for patients in class A was 5%, with an increase in the mortality advantage ratio (OR) to 2.4 [95% confidence interval (CI) 1.4-4.3] for class B patients, 3.4 (95% CI 1.9-6.0) for class C patients, and finally, for class BC patients had a significant increase in OR to 13.1 (95% CI 7.9-22.2).
Does De Bakey typing still meet current needs?
The Stanford and DeBakey system has been used to classify aortic coarctation for the past 70 years. Several new anatomic classification systems have been proposed, including the classification system proposed by Roux and Guilmet, DISSECT (duration, intimal tear, size, extent, clinical complications, thrombosis). Due to its complexity, it has not been widely used in clinical practice.
The Society for Vascular Surgery (SVS) and STS have recently proposed a new classification that includes the Stanford nomenclature. In this new SVS/STS classification, aortic coarctation is defined not by the extension of the coarctation, but by the location of the breach entry. If a patient has an entry in the aortic arch or descending aorta and the entrapment extends down to the aortic valve, it is a type B aortic coarctation.A type A aortic coarctation is defined as a coarctation in which the breach is located in the ascending aorta and proximal arch.
A European update of the Stanford classification-Type/Entry/Malperfusion (TEM) classification has recently been proposed (Figure 6).
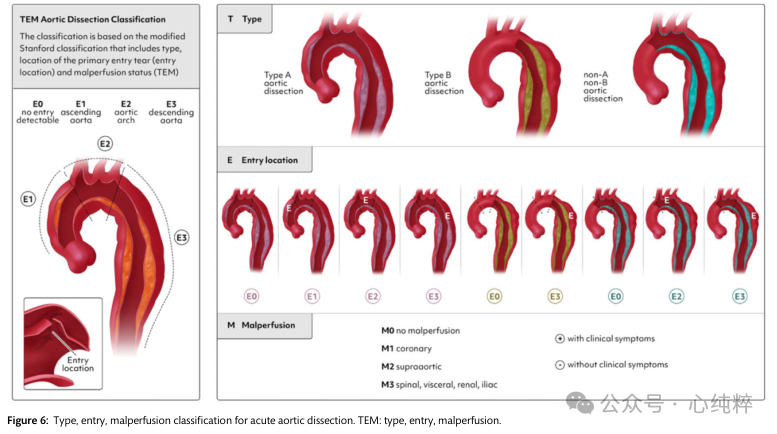
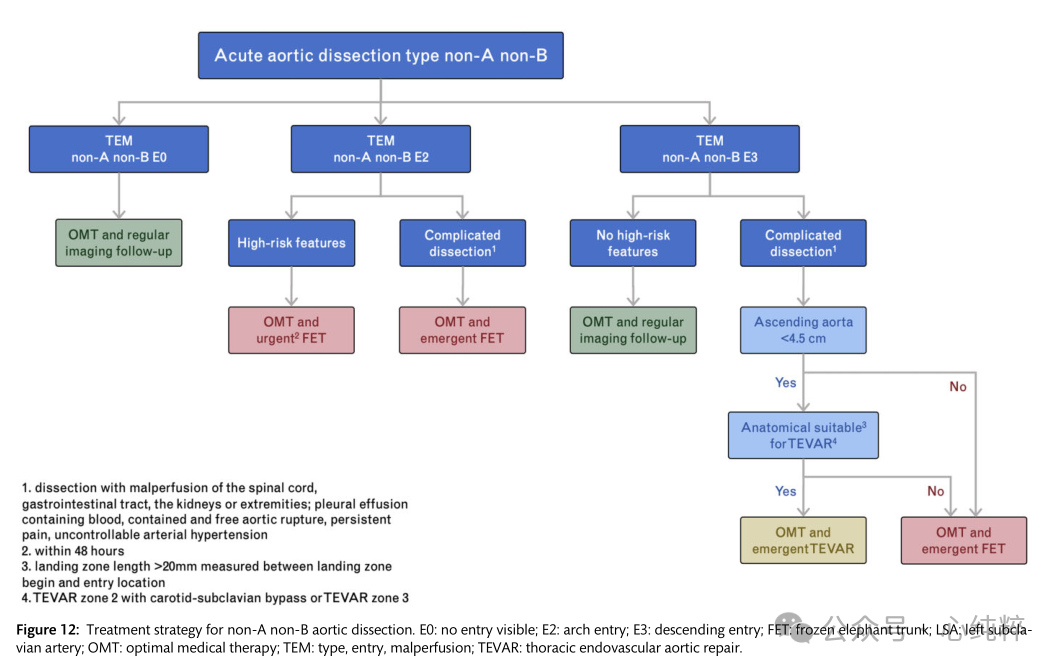
In the TEM classification of dissections, type (T) is defined by the anatomic extension proposed by the Stanford classification, not by the location of entry.The TEM provides a separate term for the first time for coarctation that includes the aortic arch but excludes the ascending aorta:non-A non-B aortic coarctation. It gives information about the location of the major entrance tear (E) and the state of malperfusion (M). If the main entrance tear is not visible, the entrance location “0” is given; if it is in the ascending aorta, ' 1 ' is written; ' 2 ' is in the arch; and “3” indicates the descending aorta (E0, E1, E2, E3). If there is no malperfusion, ' 0 ' indicates malperfusion; ' 1 ' indicates that the coronary arteries are affected; ' 2 ' if the supra-aortic vessels are stripped; ' 3 ' indicates that the viscera/kidneys and/or the lower limbs are affected (M0, M1, M2 and M3). (+) if the clinical manifestation is malperfusion and (-) if it is a radiologic finding.
Leaflet aortic valve lesions:
Aortic lesions associated with the bileaflet aortic valve (BAV) have been the subject of intense scientific debate in the past, especially regarding their pathogenesis, whether genetically determined or hemodynamically driven. The lack of a clear answer to this question has led to differences in surgical attitudes and even official guidelines. Only when the clinical and phenotypic heterogeneity of the disease began to be appreciated and systematically addressed by research, the hypothesis that these two mechanisms can coexist, with different weights for each of the different phenotypic forms, gave rise to new interest in the classification of BAV aortic lesions. However, initially different systems were proposed based on different criteria, which may have caused general confusion.
Recently, a large group of international experts reached a consensus statement on the nomenclature and classification of BAV and related aortic lesions: valves should be described as “fused,” “2-sinus,” or “partially fused” type, whereas aortic lesions should be classified as “fused,” “2-sinus,” or “partially fused” type. Aortic lesions are categorized as “radicular” (15-20% of cases, with dilatation predominantly at the sinus level), “ascending” (70-75%, with dilatation predominantly in the ascending aorta), or “extended” (70-75%, with dilatation predominantly in the ascending aorta). “Extended” (5-10% of cases, with root dilatation extending significantly into the ascending aorta and also into the proximal arch).
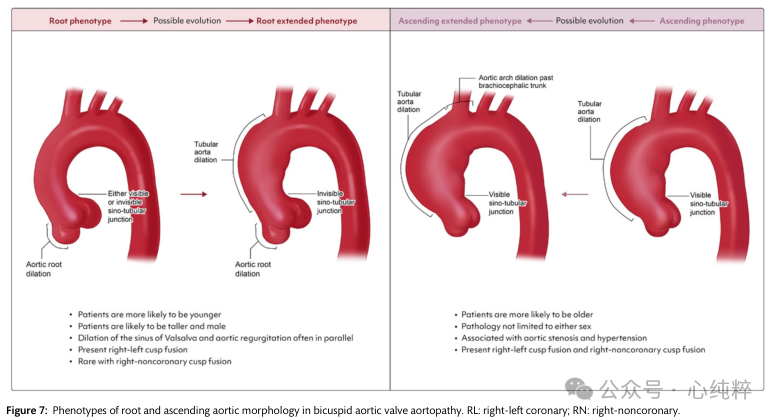
Root type: may be younger, more likely to be male, taller, often with aortic regurgitation, valves become right and left fused, less often right absent fusion.
Ascending aortic phenotype: older, less gender-specific, often with aortic stenosis and hypertension, with right absent and right-left fusion
The root phenotype shows an association with faster growth of the ascending diameter, a higher prevalence of aortic dilatation in relatives, a higher risk of acute aortic events (AAEs) during follow-up after aortic valve replacement, a higher propensity for entrapment, and potential aortopathy-associated genetic variants. Patients with root phenotypes (or root extensions) should receive stricter monitoring and earlier surgery.
A few studies have selectively addressed the ascending phenotype:in this case, aortic wall morphology and molecular changes are confined to the region of maximal flow-associated wall shear stress (WSS) (usually at the outer curvature). Growth after aortic valve replacement is very slow, and typical features associated with the extended phenotype include ascending main dilatation with valvular stenosis and right uncoronary leaflet fusion BAV.
Classification of bileaflet aortic valve lesions:The two main phenotypes are described, along with their respective typical associations with patient characteristics. The two pathogenic mechanisms of aortic lesion development (genetic variants, hemodynamic disturbances) may coexist in both phenotypes, but their respective contributions may differ. As the disease progresses over time, the extended phenotype can be an evolution of the root or ascending phenotype.
How to reduce the risk of neurologic complications:
Initial imaging plays a crucial role in assessing the extent and morphologic expression of AD as well as in determining the most appropriate therapeutic regimen.
The focus is not only on the respective aortic pathology, but also on the anatomical supra-aortic vasculature, including the cerebral supply of Willis and its structure or changes. In addition, assessment of spinal cord perfusion is very important.
Evaluation of both prior to initiating any treatment is necessary to minimize the risk of neurological complications, using CT angiography as the method of choice.
Regardless of the underlying aortic pathology, imaging the entire aorta, including the supra-aortic vessels to the femoral bifurcation, is essential. If surgery is planned on the aortic arch, the Ring of Willis should also be imaged to assess its morphology and patency. This approach allows for optimal treatment planning and, in the case of open surgery, perfusion planning and minimizes the burden of repeat examinations on the patient.
In patients with aortic pathology, anatomic variations, particularly of the aortic arch, such as carotid trunk, right subclavian artery anomalies, or left vertebral artery are common and increase the risk of postoperative neurologic deficits. Unilateral hypoplastic vertebral arteries are usually the sole source of blood supply to isolated brain regions and do not form basilar arteries as is usually the case. In addition, data show that 50% of AD patients have aortic arch abnormalities, 9% of which may be due to underperfusion caused by unilateral selective cisternal cerebral perfusion. This condition particularly affects the left posterior communicating artery, as it is hypoplastic in 35.6% of patients.
A recent meta-analysis evaluating 4 commonly used brain protection strategies showed that unilateral cerebral perfusion therapy under moderate hypothermia had better outcomes in terms of hospital mortality and stroke. Of note, this study also included bow surgery, but only 40% were full-bow procedures. However, moderate to high hypothermia with selective collateral cerebral perfusion appears to be the safest concept of cerebral protection in total arch replacement. Thus, variants of the aortic arch can significantly affect perfusion strategies and technique implementation in both open and endovascular treatment, and therefore require careful planning based on anatomic conditions.
In addition to a detailed cerebral perfusion profile, an assessment of the anatomical and functional supply of the spinal cord is essential. The blood supply is based on two fundamental interrelated components that must be evaluated prior to each procedure:(i) a 4-region concept describing the large extravertebral blood flow, including the left subclavian artery, intercostal arteries, lumbar vertebral arteries, and internal iliac arteries. (ii) Intravertebral/paravertebral arterial collateral network (CN). In addition to other factors, maximizing the preservation of these two structures is essential to prevent spinal cord ischemia. Regarding the 4-vessel region, registry data show that removal of 1 vessel region alone has a minimal impact on the development of spinal cord ischemia, whereas removal of 2 vessel regions is highly relevant, especially when combined with intraoperative hypotension.
Acute Aortic Disease-The Natural Course of the Disease and When to Intervene:
Acute aortic syndrome:
Acute aortic syndromes including aortic coarctation, IMH and PAU are life-threatening conditions that require urgent evaluation and treatment. Acute aortic syndromes can present with a variety of manifestations, including hemorrhage and varying degrees of endothelial disruption - from localized, isolated disruption in IMH to widespread, diffuse endothelial disruption in classic aortic coarctation. Regardless of subtype, all AASs require prompt diagnosis and immediate optimal pharmacologic therapy to prevent acute complications (organ ischemia or aortic dissection) and death. Pharmacologic therapy consists of strict blood pressure management to reduce aortic wall pressure while controlling pain. A target systolic blood pressure of 100-120 mm Hg and a heart rate of 60-80 bpm are recommended.
Intravenous -β-blockers prior to intravenous vasodilators (e.g.; sodium nitroprusside (Clevedipine) are effective in attenuating cardiac contractile impulses, lowering blood pressure, and reducing epicardial wall stress. Adequate invasive monitoring should be provided in the monitoring room or ICU setting.
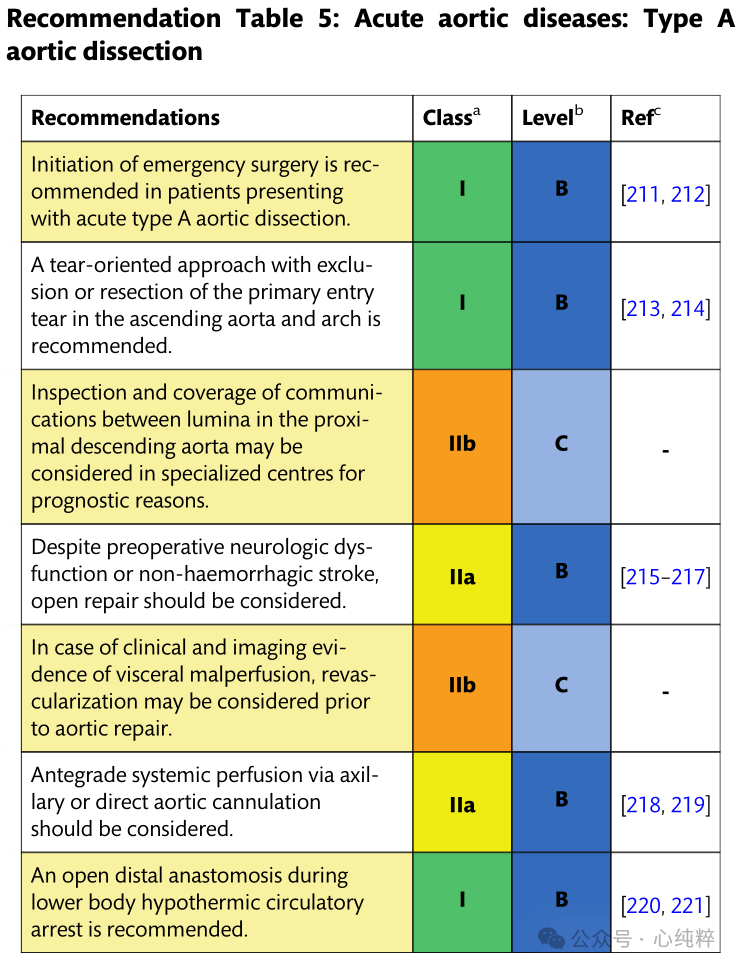
Emergency surgery is recommended for patients with acute type A aortic coarctation (IB).
Open repair should be considered despite preoperative neurologic deficits or nonhemorrhagic stroke (IIa).
If there is clinical and imaging evidence of poor visceral perfusion, revascularization may be considered prior to aortic repair (IIB).
Type A entrapment refers to entrapment from the ascending aorta. The natural course of the disease is very aggressive due to the sequelae of poor organ perfusion, acute heart failure (aortic insufficiency), and aortic dissection.
Aortic coarctation is very disorienting:Because the aorta supplies every organ in the body, aortic coarctation can manifest symptoms in any organ. A high level of suspicion for aortic coarctation is essential for emergency department staff.D-dimer blood tests are very useful in emergency situations. If D-dimer is negative, the patient does not have aortic coarctation. (D-dimer, while very sensitive, is not at all specific; it can rule out dissection, but not absolutely.) A meta-analysis showed that D-dimer >500 ng/ml increased the likelihood of identifying patients with suspected acute aortic coarctation.
If treated conservatively (without surgery), ATAAD is associated with an early mortality rate of 0.5% per hour. Surgery has been shown to be significantly superior to pharmacologic therapy. Due to improvements in diagnostic and surgical strategies, what used to be an extremely high postoperative mortality rate has decreased to 15-18% with medical advances over the past 20 years. However, each patient's condition must be evaluated by weighing the risks of surgery against all possible necessary adjuncts to optimal drug therapy. Risk scores have been established in recent years to enhance therapeutic decision-making. Conservative treatment is sometimes best for patients with significant contraindications or comorbidities. Endovascular repair in unsuitable surgical candidates is performed in a limited number of inoperable patients, primarily in a compassionate care setting. However, the aortic root is highly unreproducible in current endovascular interventions, with vulnerable aortic valve leaflets and critical coronary openings at risk.
Preoperative evaluation:
Due to type A aortic coarctation, any branch vessel of the aorta may be susceptible to poor perfusion. False lumen dilatation due to hematoma or thrombosis can lead to static obstruction of the true lumen of the aortic branches. In addition, dynamic obstruction of the aortic vasculature can lead to low-flow phenomena and subsequent malperfusion. Both mechanisms, static and dynamic, may lead to impaired end-organ perfusion. The preoperative malperfusion syndrome is associated with a high perioperative mortality rate of up to 43.4% and progressively increases with the number of poorly perfused organs.
In addition to the CT manifestations, clinical evaluation (e.g., pulsus hypoplasia, neurologic dysfunction, abdominal pain, elevated lactate, oliguria) is necessary to determine further treatment. The presence of a femoral arterial pulsation ensures blood flow along the entire aorta. In the current high-tech imaging environment, clinical evaluation cannot be forgotten or ignored. Specifically, on CT imaging, the true lumen may be reduced to a very small size; however, if the femoral pulse is preserved, blood flows (through the open window) despite the small size of the true lumen. In the modern era of hybrid operating room suites, live imaging can accurately diagnose poorly perfused organs and enhance the selection of optimal treatment algorithms.
Mesenteric malperfusion is a devastating complication associated with a nearly 5-fold increased risk of perioperative death. Considering the dismal outcomes after conventional repair, alternatives to combining visceral revascularization prior to aortic dissection repair have emerged. First hemodialysis with endovascular opening or stenting reduces in-hospital mortality and may be considered in patients with imaging and clinical evidence of mesenteric malperfusion. In the management of visceral malperfusion, this procedure carries the risk of the most serious complication of type a entrapment:intrapericardial entrapment rupture. Many individual surgeons strongly support immediate replacement of the ascending aorta (which usually, but not always, corrects intestinal malperfusion). Increasingly, the preferred approach is gut reperfusion. There is no right or wrong answer to this dilemma. Thus, in hemodynamically stable patients, it is reasonable to address visceral malperfusion through an endovascular-prioritized approach.
Coronary artery malperfusion is another dreaded complication of acute type A coarctation, with a mortality rate of up to 40%.A classification of the different types of coronary artery injuries caused by aortic coarctation was proposed in 2001 (Neri A-C), and more recently the term “coronary artery orifice endothelial tear” has been added. In patients with pericoronary detachment or complete avulsion of the coronary arteries, coronary artery bypass grafting seems to be the most appropriate treatment to restore adequate blood flow and improve coronary ischemia.
Spread of the entrapment to supra-aortic vessels, peripheral embolization, or hemodynamic compromise can lead to impaired cerebral perfusion and neurologic dysfunction. The optimal timing of surgical repair in patients with acute type A entrapment with poor cerebral perfusion is still under debate. In patients with preoperative neurologic injury (except for hemorrhagic stroke), urgent aortic repair is usually preferred to conservative treatment. In complete stroke or hemorrhagic stroke (longer duration from entrapment), immediate aortic surgery may be unwise and unrewarding. When patients are paraplegic due to spinal cord ischemia, cerebrospinal fluid drainage should be considered in preparation for ascending aortic replacement.
Despite the presence of organ malperfusion, ATAAD may be associated with acute aortic regurgitation or pericardial tamponade. In particular, poor perfusion and pericardial tamponade including shock increase operative mortality. Therefore, surgical risk must be weighed against the expected outcome. Immediate surgical treatment inevitably resolves cardiac tamponade and severe aortic regurgitation. If the pericardial tamponade opens the pericardium before central peripheral cannulation or drainage without cardiac surgery, the patient's hemodynamic status is severely compromised without delayed transport. However, resuscitation after opening the pericardium assuming the presence of severe pericardial tamponade without improvement also results in severely compromised outcomes.
Surgical treatment:
It has been well documented over the past 50 years that patients with type A aortic coarctation can be repaired urgently surgically by resection of the proximal lesion and primary rupture. Because of the unpredictable behavior of the stripped aorta (especially near the aortic valve, coronary arteries, and supra-aortic branches), rapid repair is imperative given the 0.5% per hour risk of death while waiting for surgery. Surgical repair ranges from coronary artery bypass and root replacement with valve preservation to complete resection of the ascending aorta and arch, including arch debranching techniques and partial arch replacement using FET. All techniques are influenced by a variety of factors, including the extent of the dissection, the location of the main inlet, the local level of surgical expertise, and the age and comorbidities of any given patient. To date, there are no reliable data comparing the various strategies, but the preference for an individualized strategy in high-volume clamping appears promising.
In patients undergoing aortic repair, the axillary artery should be used for arterial cannulation; hypothermic circulatory arrest combined with cisplaced cerebral perfusion and open distal anastomosis has yielded favorable results. In addition, a breach-directed approach, including resection or exclusion of the primary breach, is recommended. Consideration should be given to examining the proximal descending aorta during hypothermic circulatory arrest. Implantation of a FET may be considered in cases where the rupture is in the distal arch or proximal descending aorta.As an alternative, postoperative TEVAR can be used to cover the rupture and optimize true luminal perfusion.The combination of these three concepts (axillary perfusion, circulatory arrest with cerebral perfusion, and descending aortic evaluation/treatment) enhances true lumen perfusion, promotes false lumen thrombosis, and reduces the risk of late aortic complications or reoperation.The recommended therapeutic range for type A aortic coarctation is illustrated in Figure 11.
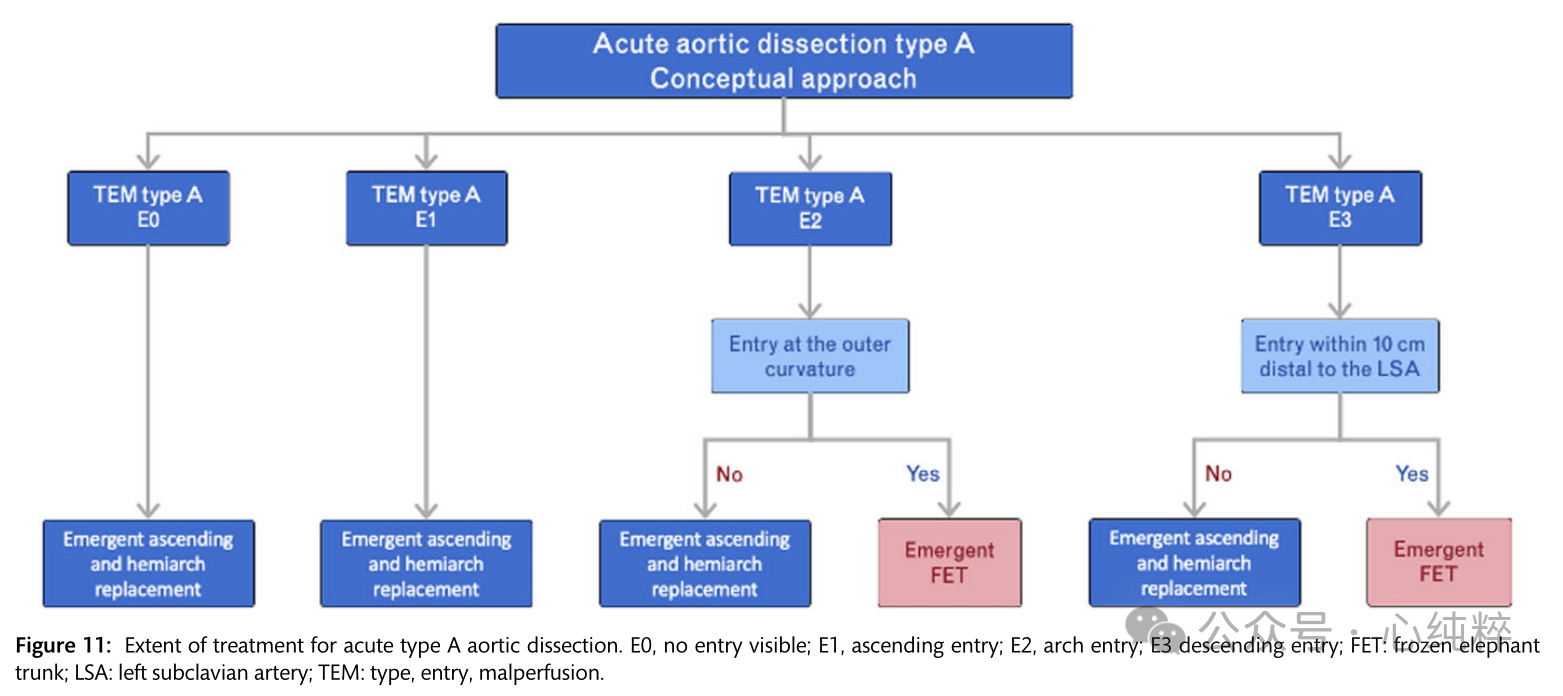
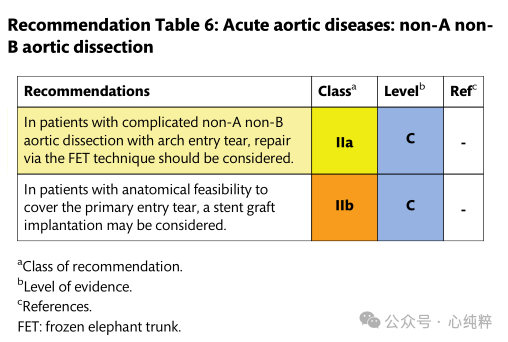
Non-A non-B aortic dissection:
The combination of aortic arch and descending aortic intima-media rupture without stripping of the ascending aorta has not been considered in past Stanford or DeBakey classifications. The new SVS/STS classification describes TBAD (type B dissection) as any aortic dissection with a primary rupture distal to zone 1 or more.
Thus, Stanford's classification of this particular but rare anatomic pattern has been described by various authors as non-a non-b. Conservative treatment is associated with a high mortality rate due to early malperfusion and aortic dissection, paving the way for surgical and endovascular treatment within 14 days of symptom onset.
Few data are available on the outcomes of the nona-nonb sandwich subgroup. Early reports seemed to favor open surgical resection of the entrance tear of the arch, similar to the management of type A entrapment, with the goal of decompression and exclusion of the breach and subsequent false lumen expansion. In contrast, however, medically managed patients tend to present with progressive enlargement, rupture, or the need for late extensive replacement surgery. Indeed, the largest registry in the world reports nona nonb anatomic outcomes, with 19% of patients undergoing open arch replacement with a mortality rate of 31% or 25% undergoing endovascular access with a mortality rate of 14.3%, which is similar to the 13.9% mortality rate for the majority (>50%) of patients who undergo medical management; regardless of treatment, the most common complication is stroke.
Recent experience in 39 patients over a 20-year period suggests either a hybrid endovascular approach (combined vascular debranching or bypass surgery), which is still associated with an overall mortality rate of 27%, or FET for total arch replacement in selected young patients.
Similarly, a report from China showed that 79 patients with open surgical arch replacement and FET had an operative mortality rate of 5.1% and a serious neurologic event (stroke/paraplegia) of 6.3%, but a 1-year survival rate of 82.3%.
Given the complexity of arch replacement procedures associated with FET, even staged debranching and endovascular interventions, new strategies are emerging. A modified surgical technique that involves the use of a scalloped (windowed to maintain supra-aortic blood flow) stent inserted in passing through a median sternotomy is used to close the primary breach.
Overall, there is no consensus on the optimal treatment of acute or subacute nona-nonb aortic coarctation; simplified and less invasive hybridization procedures/endovascular access are emerging and may offer advantages over complex and complete arch replacement. Treatment recommendations for non-a non-b aortic coarctation are shown in Figure 12.
Type B aortic coarctation:
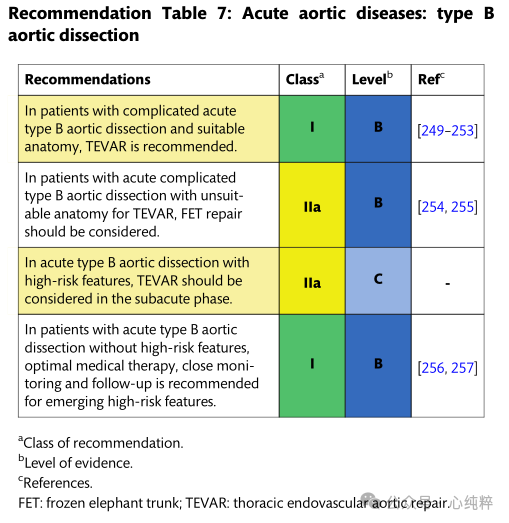
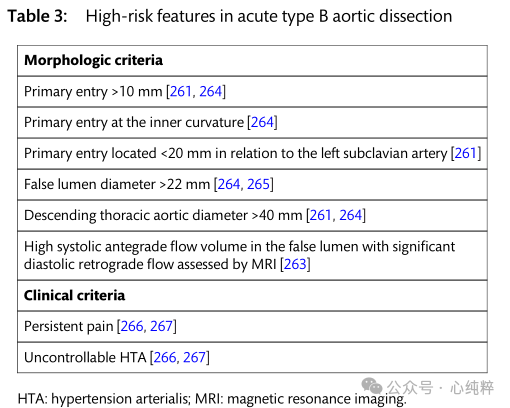
Acute type B aortic coarctation accounts for 30-38% of all aortic coarctation. Overall, the hospitalized mortality rate for acute TBAD has not changed over the past 20 years. The International Registry of Acute Aortic Coarctation database reported a mortality rate of 14.1% for patients with acute type B coarctation between 2010 and 2013. In contrast to type A aortic coarctation, clinical presentation and imaging features directly influence treatment approach. Identification of high-risk features is a prerequisite for the choice of intervention or surgical repair.

Intramural hematoma (IMH) of the aorta):
Intramural hematomas are characterized by > 5 mm of localized bleeding within the aortic wall, which may or may not be associated with intimal rupture. Intramural vessel rupture, PAU, or trauma are thought to be the primary pathologic mechanisms of IMH. Based on improved high-resolution CT scanning imaging techniques and clinical experience, there is growing evidence that localized intimal disruption (categorized as ulcer-like projections) may lead to IMH as intimal disruption occurs progressively over time.
However, there are other explanations that ulceration (ULP) may be caused by IMH, as intimal destruction may occur in the acute or subacute phase and ULP occurs progressively after focal intimal destruction in IMH.IMH is located predominantly in the descending aorta (60-70%) in type B IMH, rather than in the ascending aorta (30%) in type A IMH or the aortic arch (10%).
Acute type A IMH can present with life-threatening disease. Aortic events are frequent in the first 7 days due to spread of the hematoma within the aortic wall.33-40% of type A IMHs progress to type A aortic coarctation and even rupture on admission in up to 18% of patients. In case of complications such as malperfusion and rupture, urgent surgical treatment is recommended, which is the same as the treatment of type A aortic coarctation.
Based on imaging data and clinical series, further risk factors for a malignant course of type a IMH have been identified and a better risk assessment score has recently been proposed. Given the high mortality rate in patients with high-risk features, emergency surgical repair is preferable to pharmacologic therapy alone.
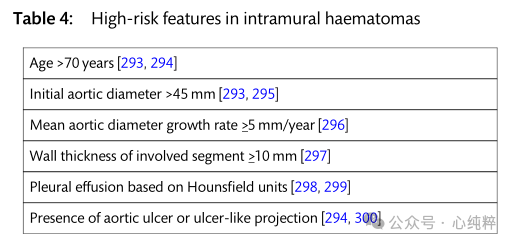
In patients without high-risk features, optimal pharmacological treatment helps stabilize the patient, and in these patients, close imaging follow-up is mandatory to detect early progression and re-evaluate the individual's conservative treatment approach. The literature suggests that rates of progression to surgical repair or intervention are as high as 30-40% after initial conservative treatment. If progression to aortic coarctation or increased aortic diameter as well as increased hematoma thickness occurs, rapid surgical repair is required.
In patients with acute type A IMH due to descending aortic tears or even type B coarctation, TEVAR has been reported in small clinical series as an alternative treatment to open surgery or pharmacologic therapy alone. In selected patients with ascending aortic diameter <50 mm and IMH thickness <10 mm, TEVAR was associated with a 5-year survival rate of 98%. In the absence of larger comparative studies, this treatment strategy remains controversial and must be carefully weighed on an individual basis in selected patients.
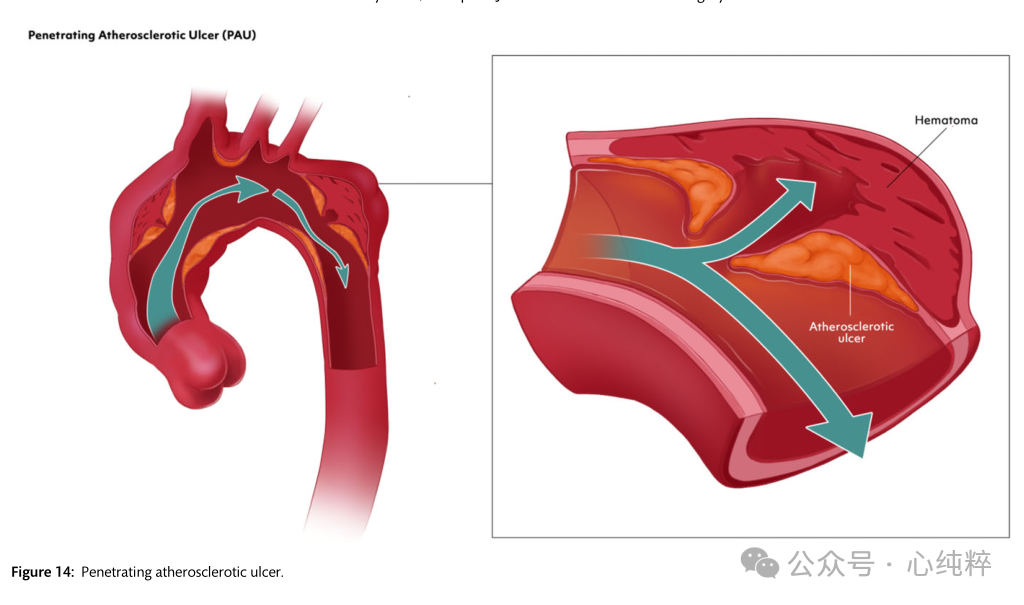
Penetrating atherosclerotic ulcer:
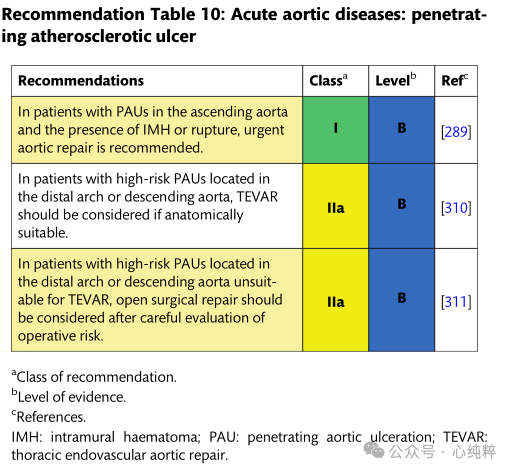
Penetrating atherosclerotic ulcers are defined as atherosclerotic lesions of the aortic wall originating in the intima and progressing toward the midma. Based on the underlying disease, patients have a high atherosclerotic burden, are usually older, and have other comorbidities. In such a clinical context, the procedural risk of endovascular or open surgery should be weighed against frailty, comorbidities, and life expectancy.
Clinical presentation and anatomic features guide different treatment strategies. Most PAUs are asymptomatic and are discovered incidentally on imaging for other indications; they are located primarily in the descending aorta.
Due to the ulcerative and degenerative nature of the disease, PAUs can be associated with restrictive IMH and have a high risk of progressing to aortic coarctation or rupture, especially those located in the ascending aorta.Therefore, symptomatic PAUs or PAUs located in the ascending aorta with high-risk features should be urgently surgically repaired. For patients with PAUs located away from zone 0, treatment depends on the presence of complications-rupture, IMH or aortic dissection, persistent pain, or pleural effusion. In addition, the high-risk features of any PAU with a malignant course should be evaluated.
Traumatic aortic injury:
Blunt or penetrating trauma can cause aortic injury. The rapid deceleration events of blunt trauma result in rapid movement of the aortic segment relative to a fixed anatomic point. Blunt thoracic aortic injury (BTAI) usually occurs in the isthmus of the aorta and less frequently elsewhere.BTAI usually affects young males and is associated with a high mortality rate before the patient reaches the hospital. The following are BTAIs in the isthmus of the aorta.BTAIs involving the ascending aorta or aortic arch are rare and should be treated on an individual basis in the aortic arch group.BTAIs are categorized into 4 grades:Grade I: intimal tear; Grade II: intramural hematoma; Grade III: pseudoaneurysm; and Grade IV: rupture.BTAIs are classified into 4 grades:Grade I: intimal tear;Grade II: intramural hematoma;Grade III: pseudoaneurysm;Grade IV: rupture.
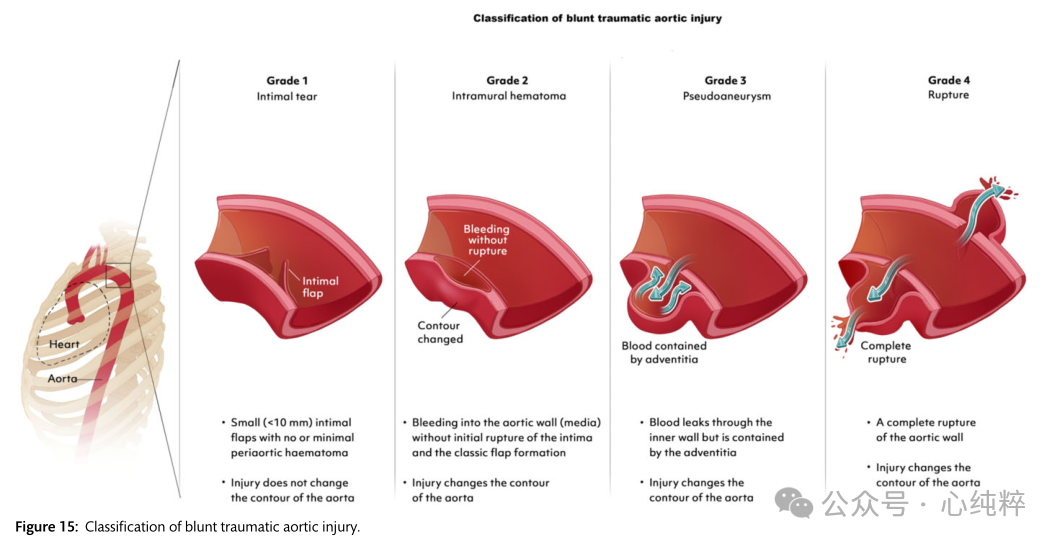
Depending on the severity of the lesion, symptoms range from completely asymptomatic or nonspecific pain to hemorrhagic shock. Therefore, it is critical that physicians should base their clinical assessment on the Progressive Trauma Late Life Support Guidelines on the preclinical situation and the type of traumatic event. Obvious clinical signs may include active bleeding, pulsatile or expanding hematoma, wound swelling or tremor, loss of distal pulse, or ischemia of the extremities (pain, pallor, paresthesia, hypothermia). However, the diagnosis needs to be confirmed on contrast-enhanced CTA imaging.
Management of BTAI depends on the severity of the lesion and therefore on the BTAI grading system. In stable patients, transfer to a high-volume specialized trauma center is preferable for optimal results.
Endovascular aortic repair is the treatment of choice if appropriate dissection is present, and grade I BTAI lesions can be treated nonsurgically (e.g., maintaining systolic blood pressure <100 mmHg and heart rate <100 bpm) with close follow-up.
Grade II BTAI lesions include lesions with and without high-risk imaging features, and are characterized by: posterior mediastinal hematoma >10 mm, lesion-to-normal aortic diameter ratio >1.4, mediastinal hematoma causing a placeholder effect, pseudo-constriction of the aorta; left hemothorax; involvement of the ascending aorta, aortic arch, or great vessels; or aortic arch hematoma. For grade II BTAI lesions with high-risk imaging features, a TEVAR treatment strategy should be considered. Conversely, for lesions without these high-risk imaging features, nonsurgical treatment and close follow-up may be preferred.
Grade III-IV lesions should be treated with TEVAR surgery.


