Aortic root aneurysm is a potentially life-threatening condition that can lead to aortic dissection and is often associated with genetic syndromes such as Marfan's syndrome (MFS). Although studies of animal models of MFS have provided valuable insights into the pathogenesis of aortic root aneurysms, the understanding of the transcriptomics and epigenetic profiles of human aortic root tissues remains inadequate, a limitation that has hampered the development of effective targeted drugs.
Recently, the team of Zhou Zhou, Shu Chang and Luo Mingyao from Fu Wai Hospital, Chinese Academy of Medical Sciences, in collaboration with Wang Kai from Peking University Medical School, published a paper titled “Single-Nucleus Multiomic Analyses Identifies Gene Regulatory Dynamics of Phenotypic Modulation in Human Aneurysmal Aortic Root”. Xuanyu Liu, an associate researcher at Fu Wai Hospital, Chinese Academy of Medical Sciences, and Qingyi Zeng, a doctoral student, are the co-first authors of the paper. In this study, we constructed the first single-cell nuclear multi-omics profiles of gene expression and chromatin accessibility in human aortic root tissues under healthy and MFS conditions. This study provides new insights into transcriptional regulation and spatial dynamics during phenotypic regulation of human aortic root smooth muscle cells.
Article Profile
Chinese title: Dynamic changes in gene regulation during phenotypic regulation of human aortic root aneurysms revealed by single nucleus multi-omics analysis
Published by: Fu Wai Hospital, Chinese Academy of Medical Sciences, School of Basic Medical Sciences, Peking University, China
Published: 2024.03
Journal: Advanced Science
Impact factor: 15.1
Subjects: Aneurysmal aortic root tissue from MFS patients (n=6); Normal aortic root tissue from heart transplant recipients with CTRL (n=6)
Histology type: 10X Genomics Chromium snRNA-seq+scATAC-seq (n=4 per group), 10X Visium Spatial (n=2 per group)
研究思路
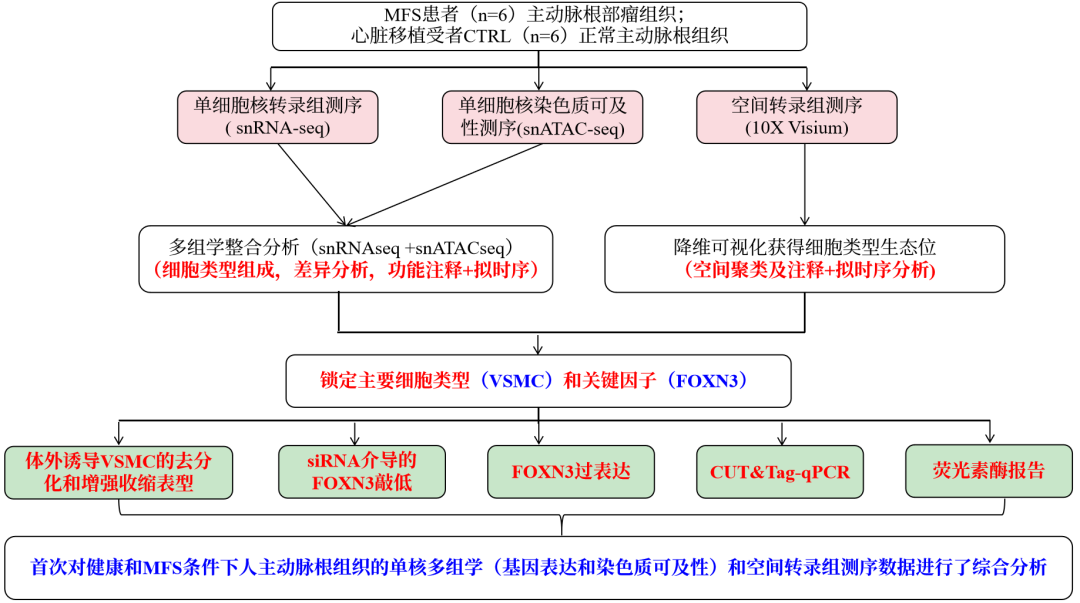
Key findings
Unsupervised clustering of all nuclei was able to identify the major cell types in the aorta, including vascular smooth muscle cells (VSMCs), fibroblasts (FBs), mural cells (Mural), vascular endothelial cells (VECs), lymphatic endothelial cells (LECs), adipocytes (Adipocyte), myeloid cells (Myeloid) and lymphoid cells ( Lymphoid). Notably, VSMC accounted for 76.9% of all nuclei, which is consistent with the expected cellular composition of human aorta.VSMC can be further subdivided into three subpopulations. Using single-nucleus multi-omics data, cell type-specific expression patterns and gene regulation patterns in the human aortic root were identified. And transcription factor (TF) binding motifs enriched for cis-regulatory elements (cCRE) specifically in each cell taxon were identified. Functional enrichment of cell type-specific cCREs was inferred using the peak functional annotation software GREAT.
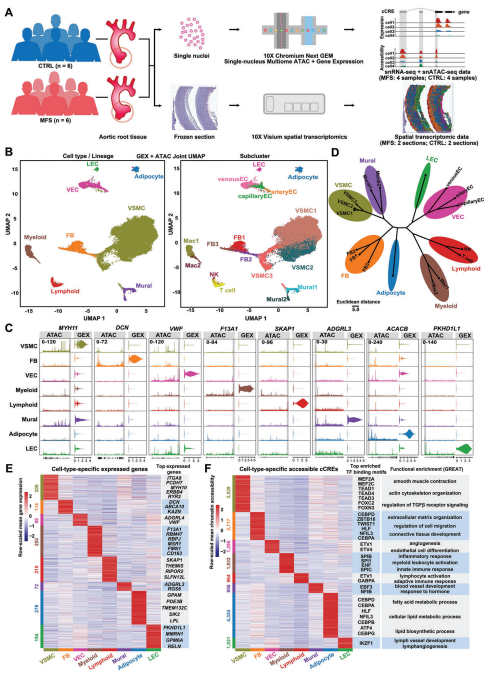
Figure 1 Cell type-specific expression patterns and gene regulation patterns in human aortic root tissue under healthy and MFS conditions.
The two groups showed a high overall similarity in cell type composition, a finding consistent with previous mononuclear studies on ascending aortic aneurysms. Nonetheless, VSMC2 (VSMC subpopulation) accounted for a significantly higher proportion of MFS samples compared with controls, reflecting its association with MFS. There were no significant differences in cellular composition between the two groups, but there were significant differences in gene expression and chromatin accessibility, particularly in VSMCs, where TF-binding motifs with significantly altered activity were detected.GO functional enrichment analysis demonstrated that under MFS conditions, upregulated DEGs in VSMCs were enriched for functions that are associated with VSMC de-differentiation or phenotypic switching, such as vasculature system development, positive regulation of cellular differentiation , extracellular matrix remodeling and TGFβ receptor signaling pathway, and DA cCREs enrichment was largely consistent with DEGs.The results of GSEA enrichment analysis showed that up-regulated signaling pathways in VSMCs were identified under MFS conditions, such as NOTCH3, RhoGTPase, ROBO receptor, TGFβ receptor complex and receptor tyrosine kinase signaling pathway.
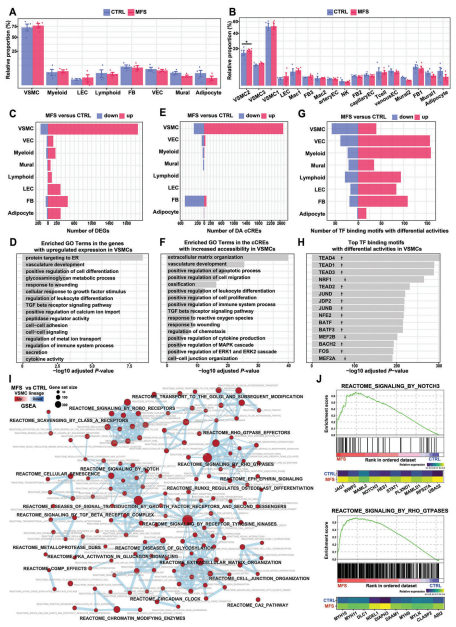
Figure 2 Altered cellular composition and cell type-specific regulatory changes in MFS versus CTRL samples revealed by single nucleus multi-omics analysis
Analysis of the single nucleus data allowed us to reveal the expression pattern of highly plastic VSMC, including three isoforms.VSMC1 expresses high levels of RYR2, which encodes an intracellular Ca2+ release channel associated with contractility of VSMC and typical contractile markers such as ACTA2 and CNN1. These findings suggest that VSMC1 represents a contractile VSMC phenotype.VSMC2 expresses high levels of genes that characterize the regulation of VSMC, such as COL8A1 and SERPINE1.Notably, VSMC3 also expresses genes that characterize the regulation of VSMC (e.g., COL8A1) but has a different expression profile than VSMC2 (e.g., high expression levels of LAMA2 and CFH at high expression levels). Reanalysis of publicly available single-cell datasets of aortic root tissue from patients with MFS also supports the idea that VSMC3 represents a specific state of VSMCs rather than fibroblasts.Proposed temporal analyses of VSMCs reveal that VSMC1 and VSMC3 may represent two extremes of developmental differentiation. By single-molecule fluorescence in situ hybridization (smFISH), the presence of these two subpopulations s human aortic root tissues was confirmed and showed differences in spatial distribution: the RYR2-high VSMC1 cells were located predominantly in media close to the outer membrane, whereas CFH-high-expressing VSMC3 cells were preferentially located in media close to the inner membrane. As expected, the relative proportion of VSMC1 cells decreased and the proportion of VSMC2 cells increased under MFS versus CTRL conditions. In addition, dynamic changes in gene expression and chromatin accessibility during the regulation of the VSMC phenotype were revealed by the identification of genes or cCREs whose expression or accessibility changed over the simulated time. Functional enrichment analysis of these genes or cCREs revealed phenotypic regulation of VSMCs from a contractile phenotype to a synthetic phenotype with increased proliferation and migration. The expression of contractile phenotype markers (e.g., RYR2 and MYH11) progressively decreased with differentiation trajectory, whereas the expression of markers regulating the synthetic phenotype (e.g., COL8A1, SERPINE1, and TNFRSF11B) increased. Notably, some TF genes, such as FOXN3, BACH1, BACH2 and TEAD1, were dynamically expressed on the differentiation trajectory, reflecting their potential role in phenotypic transition. Similarly, we identified cCREs that change dynamically with differentiation trajectories. for example, intergenic cCRE accessibility (chr11: 12552464-12553207) was positively correlated with TEAD1 expression, suggesting that this regulator may be an enhancer.
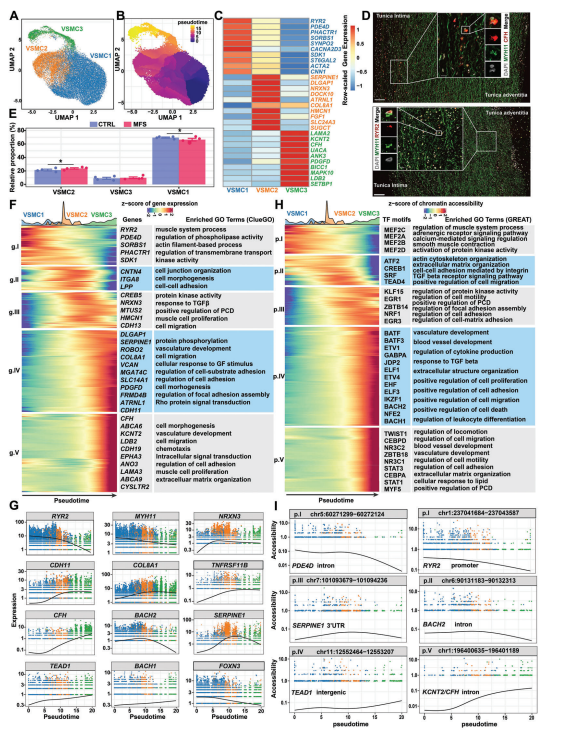
Figure 3 Dynamics of gene expression and gene regulation during phenotypic regulation of VSMC in the human aortic root
To identify potential key regulators driving the regulation of VSMC phenotype, single-cell weighted gene co-expression network analysis (scWGCNA) was performed. Nine gene co-expression modules were identified in VSMC. By comparing the expression scores of the modules in different conditions, two major modules were detected to be significantly altered in MFS: module M7 had a significantly lower expression score in MFS conditions than in CTRL conditions, whereas module M9 had a significantly higher expression score in MFS.The expression distributions in the UMAP projections showed that VSMC expressing module M7 usually overlapped with VSMC1, and that expressing module M9 VSMC usually overlapped with VSMC2 and VSMC3. Consistent with this, the Hub genes of M7 include markers for contractile VSMC (e.g., PDE4D and RYR2), whereas the Hub genes of M9 include markers for regulatory VSMC. Notably, the core TFs of M7 and M9 included TFs that were dynamically expressed in the proposed chronological analysis, such as BACH2, FOXN3, and TEAD1.Taken together, the co-expression modules of genes associated with contractile or regulatory phenotypes of VSMC were identified.
Next, potential key regulators driving VSMC phenotypic regulation were prioritized by integrating the results of multiple analyses, including significant differences in TF gene expression, regulator (TFs and their predicted targets) expression activity, and TF-binding motif activity, as well as the results of the proposed temporal sequencing analysis. A total of 11 TFs were obtained, including TGFβ pathway-associated regulators (SMAD3 and TWIST1), stress-responsive regulators (FOS, JUN, and JUNB), BACH-family regulators (BACH1 and BACH2), calcein (RUNX2), and others (TEAD1, FOXN3, and CREB1.) TEAD1 is a previously reported VSMC contractile gene expression repressor in the list of candidate key regulators and is supported by multiple lines of evidence. Notably, FOXN3, which is predominantly expressed in contractile VSMC, was the only regulator whose expression and regulatory activity was significantly reduced under MFS and CTRL conditions. Chromatin accessibility data showed that FOXN3's exhibited higher chromatin accessibility in VSMC's in the MFS group compared to the CTRL group.Westernblot assays confirmed that protein levels of FOXN3 were significantly reduced in aortic root tissue culture media from MFS patients.Functional enrichment of the FOXN3 regulator suggests that FOXN3 may be targeted in association with contractility, VSMC phenotype regulation, and aortic aneurysm pathogenesis, such as “vascular smooth muscle contraction”, “actin cytoskeleton regulation”, and “adhesion plaques”. Taken together, our data reveal potential key regulators, such as FOXN3, that may drive the phenotypic regulation of VSMC and the pathogenesis of aortic root aneurysms.
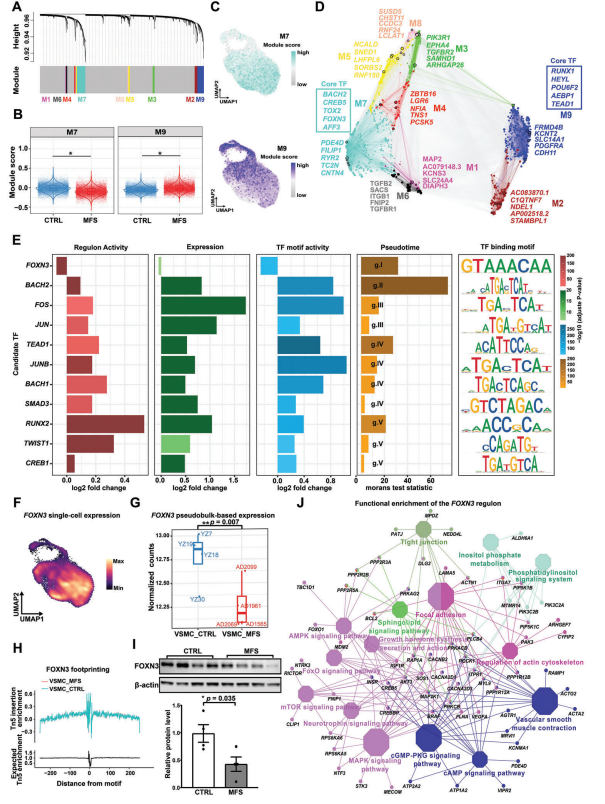
Figure 4 Potential key regulators that may drive VSMC phenotypic regulation and aortic root tumor pathogenesis
VSMC may exhibit phenotypic differences in the aortic wall due to differences in factors such as mechanical load and cellular microenvironment. Nine spatial spot sets were identified by spatial clustering and annotation, and cell types were annotated according to their expression characteristics. Notably, the spot sets annotated as VSMC were located in the UMAP clustering map, forming a distribution of phenotypic variation from highly contractile VSMC (SC4) to unregulated contractile VSMC (SC0) to regulated VSMC (SC1). These spot sets show a radial distribution from the outer layer (outer side) to the inner layer (inner membrane side) on midmembrane sections. Single-cell annotation based on single-cell transcriptome data confirmed the spatial distribution of the three VSMC isoforms on tissue sections, with the contractile subcluster VSMC1 predominantly located in the outer layer of the midmembrane and the regulatory subclusters VSMC2 and VSMC3 predominantly located in the inner layer. The results of the proposed temporal analysis indicated that for VSMC spatial points (SC4, SC0, and SC1), there was a shift from the outer layer (unregulated contractile) to the inner layer (contractile), which also represented the expression dynamics during the regulation of the VSMC phenotype. Regulatory VSMC is not only associated with disease state but also with spatial location.

Figure 5 Spatial transcriptome showing the spatial expression pattern of VSMC in the mesenchymal region of the human aortic root
6, FOXN3 can act as a key regulator in maintaining the contractile phenotype of human aortic VSMC
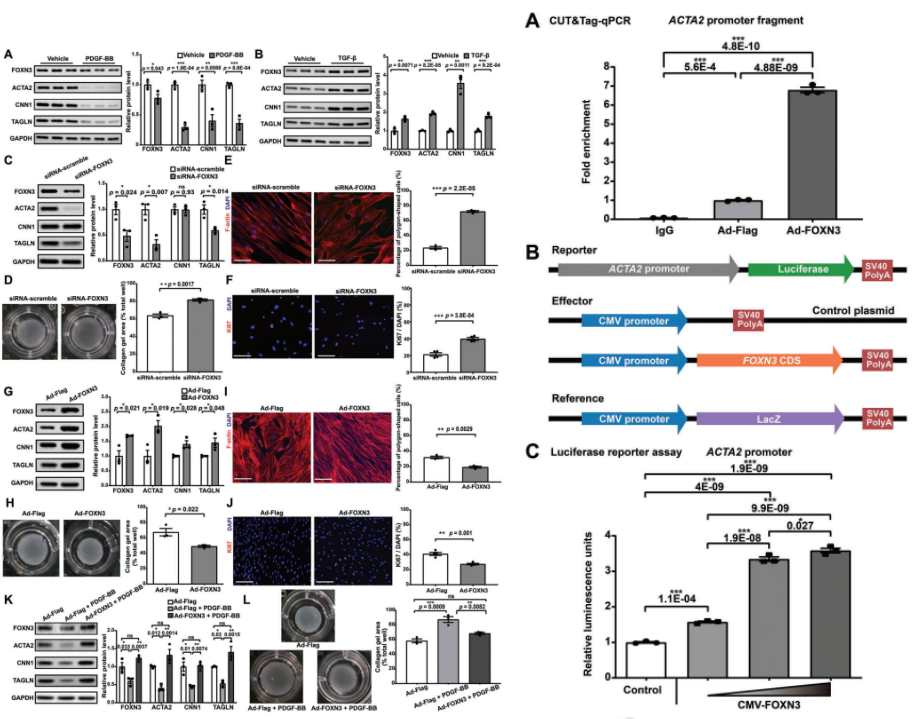
The study continued to validate the functional role of the candidate key regulator FOXN3 in human aortic smooth muscle cells (HASMC) in vitro. The results showed that FOXN3 expression was positively correlated with contractility in HASMCs. Next, FOXN3 knockdown was performed and the protein levels and mRNA expression of contractile markers were significantly reduced as well as the contractility of HASMCs was significantly reduced. In addition, FOXN3 knockdown significantly increased the percentage of polygonal cells (i.e., regulated VSMCs and Ki-67-positive cells (i.e., proliferating cells), suggesting that FOXN3 knockdown promotes phenotypic regulation of HASMCs toward a dedifferentiated phenotype. In addition, analysis of bulk RNA-seq data revealed that genes down-regulated by FOXN3 knockdown were significantly enriched in functions related to muscle contraction.FOXN3 overexpression experiments revealed a significant increase in protein levels and mRNA expression of contractile markers, and a significant enhancement of contractility. The percentage of polygonal cells and Ki-67-positive cells was significantly reduced, suggesting that FOXN3 overexpression enhanced the contractile phenotype of HASMCs. Furthermore, experimental evidence showed that FOXN3 overexpression attenuated the phenotypic regulation of HASMCs induced by PDGF-BB stimulation.
ACTA2 is not only a marker for contractile VSMCs, but also a key component of contractile function in VSMCs, and mutations in ACTA2 lead to familial thoracic aortic aneurysms and aortic coarctation. Our mononuclear data support that ACTA2 may be a target of FOXN3. To verify this, CUT&Tag-qPCR was performed in HASMC using the FOXN3 antibody. the FOXN3 antibody significantly enriched the ACTA2 promoter fragment, whereas IgG used as a negative control did not show specific amplification. Moreover, enrichment of the ACTA2 promoter fragment was further increased upon FOXN3 overexpression. These results demonstrate the binding of FOXN3 to the ACTA2 promoter in HASMC. In addition, luciferase reporter gene assay showed that FOXN3 concentration was positively correlated with the activity of the ACTA2 promoter, suggesting that FOXN3 has a regulatory effect on the activity of the ACTA2 promoter. Taken together, these results suggest that FOXN3 may act as a key regulator in maintaining the contractile phenotype of human aortic VSMC by targeting the ACTA2 promoter, and thus may serve as a potential target for the treatment of aortic aneurysms.
Bibliography.


