

The Fabulous® Postmarketing Clinical Study is a multicenter, retrospective, real-world study evaluating the safety and efficacy of the Fabulous® Thoracic Aortic Stent System for the treatment of Stanford Type B aortic dissection. Professor Weiguo Fu of the Department of Vascular Surgery, Zhongshan Hospital, Fudan University, shared the latest findings at the 50th International Vascular and Endoluminal Vascular Congress (VEITH2023), which was held in New York City, New York, U.S.A., on November 15, 2023, local time.
Fabulous® is the only thoracic aortic stent system in China that consists of a proximal coated stent and a distal bare stent, and its therapeutic principle is to renew the bare stent at the distal end of the coated stent to treat Stanford type B aortic coarctation. The results show that it has significant benefits in promoting false lumen thrombolysis, distal true lumen remodeling and improving poor visceral vascular perfusion.
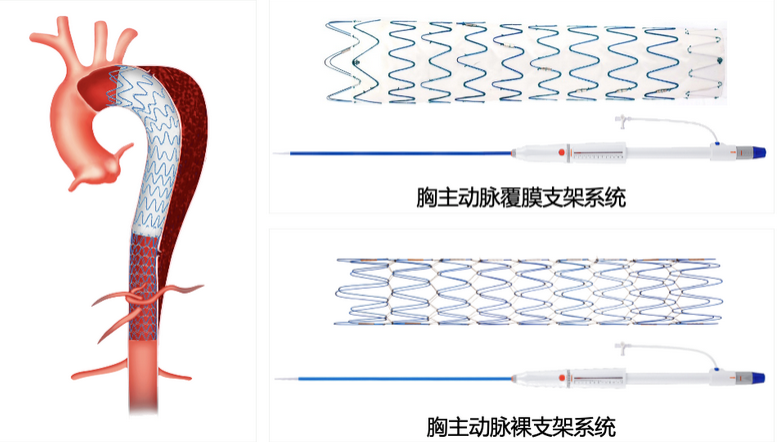
Research Program
The plan is to enroll real-world data from 260 patients at eight centers to study the effectiveness of Fabulous® for aortic remodeling. Currently 44 cases have been collected from two centers (Zhongshan Hospital of Fudan University and the First Hospital of Harbin Medical University).
Preliminary Results
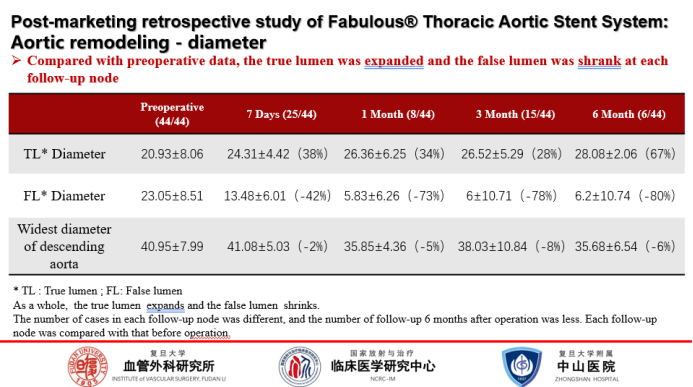
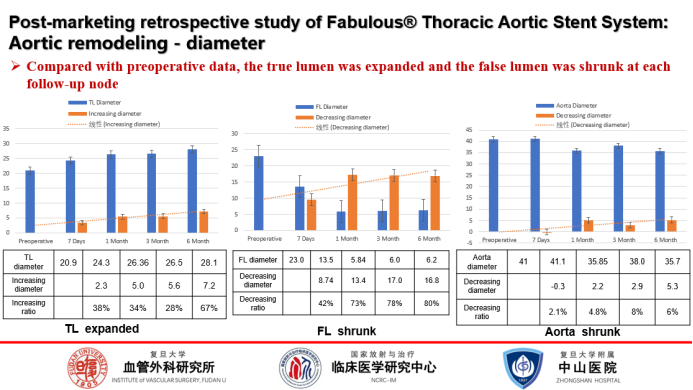
Aortic remodeling:
In comparison with the preoperative period, the true lumen had a significant enlargement of 38% at 7 days postoperatively; the false lumen shrank by 42% at 7 days postoperatively and by 78% at 3 months postoperatively; and the maximum diameter of the aorta became progressively smaller over time.Fabulous® has shown better positive remodeling early on and looks forward to more forward data support.
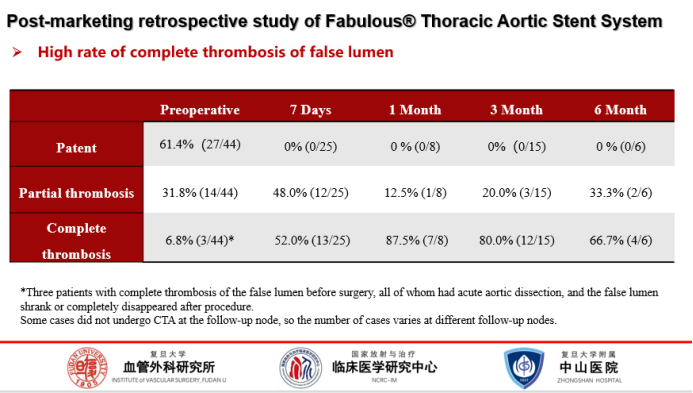
Pseudocavity thrombosis:
All follow-up nodes were compared with the preoperative period, and the rate of complete thrombosis of the false lumen was 52% at 7 days postoperatively and 80% at 3 months postoperatively.The Fabulous® stent promotes pseudoluminal thrombosis significantly.
The rate of complete thrombosis of the prosthetic lumen at 7 days, 30 days, and 6 months after Fabulous was significantly better than in comparable international studies when compared to comparable products.
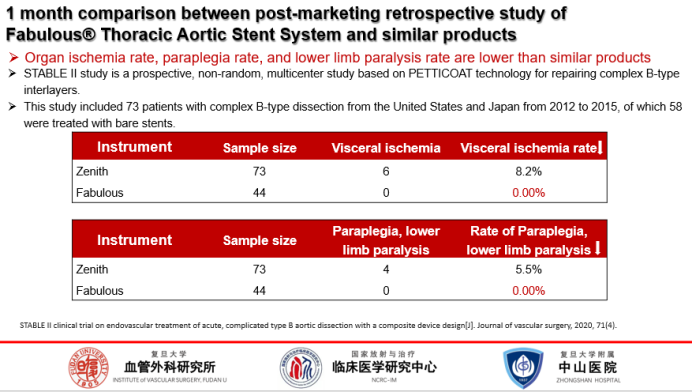
Rates of organ ischemia, paraplegia, and lower limb paralysis:
In the 30-day postoperative results, Fabulous had a lower rate of organ ischemia, paraplegia, and lower extremity paralysis than its counterparts.
Case Sharing
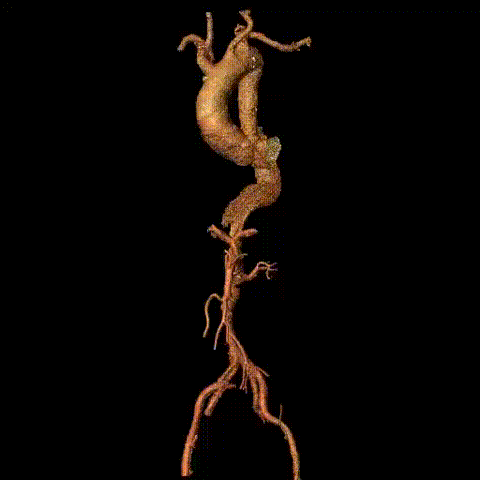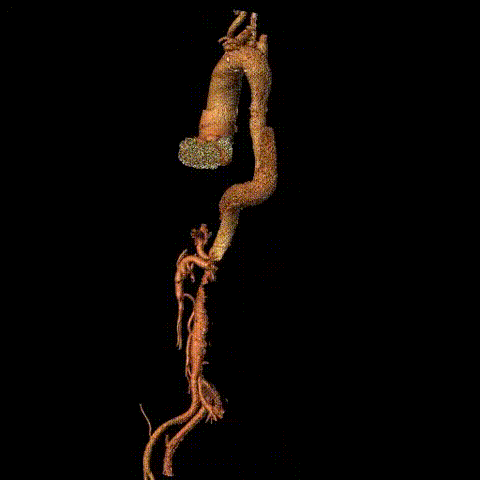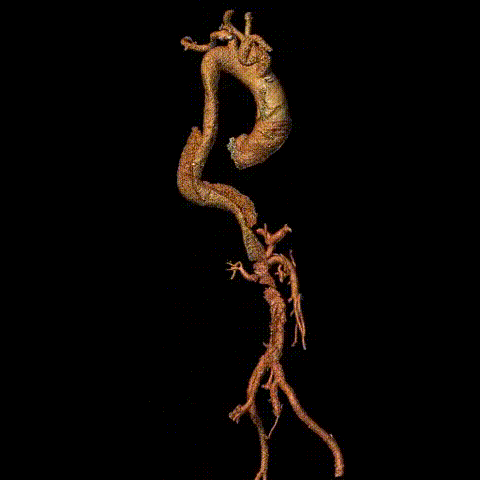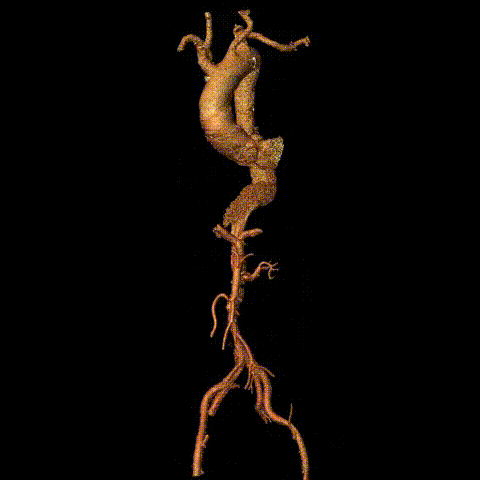
Preoperative CTA reconstruction
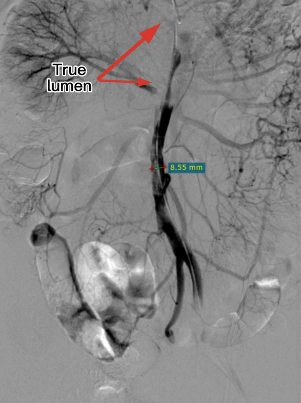
Intraoperative imaging showed no visualization of the right iliac artery, slow flow in the left iliac artery, and no visualization of the inferior abdominal aorta/
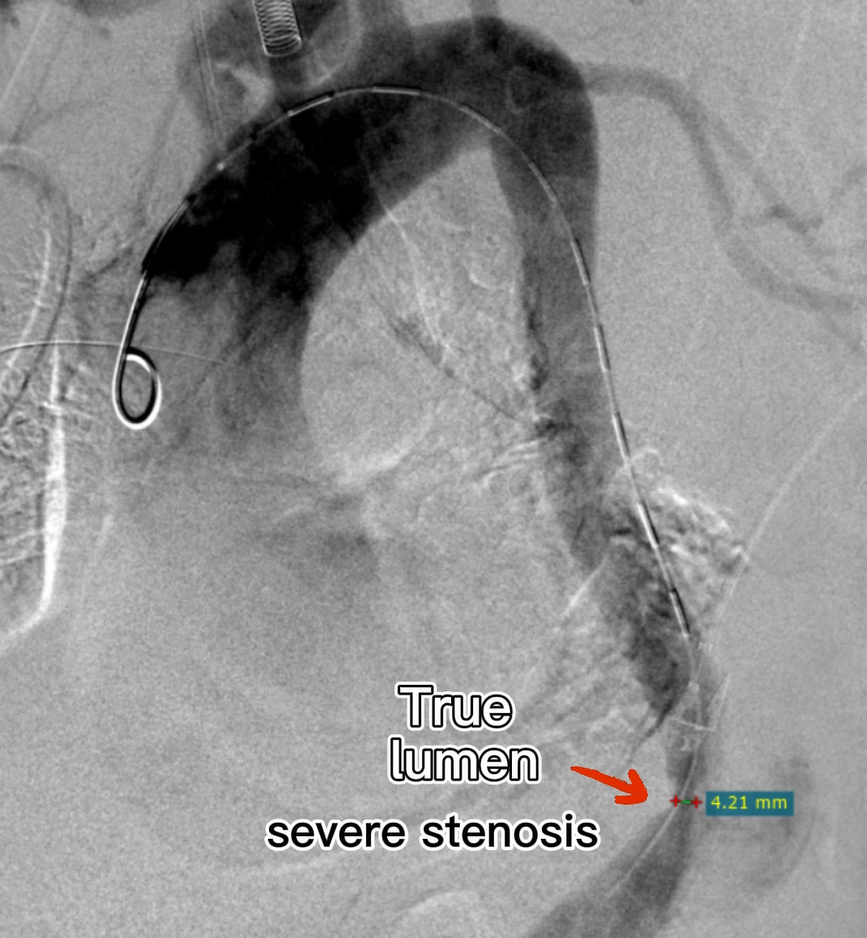
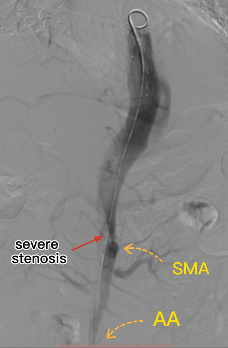
The first laminar stent was placed and the true lumen of the distal visceral area was not opened.
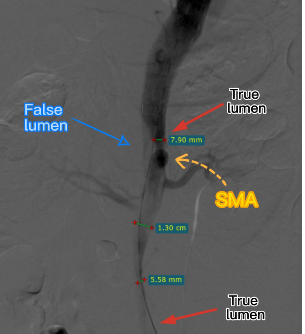
The first bare stent was released to overlap the distal end of the laminated stent, and the distal true lumen remained unopened.
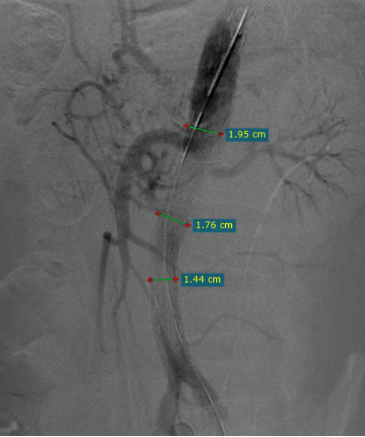
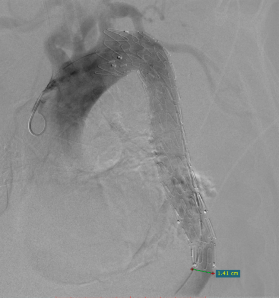
The second bare stent was released and blood supply to the true lumen of the visceral area was restored.
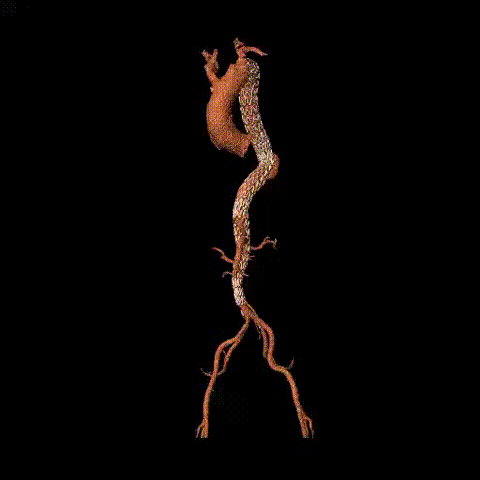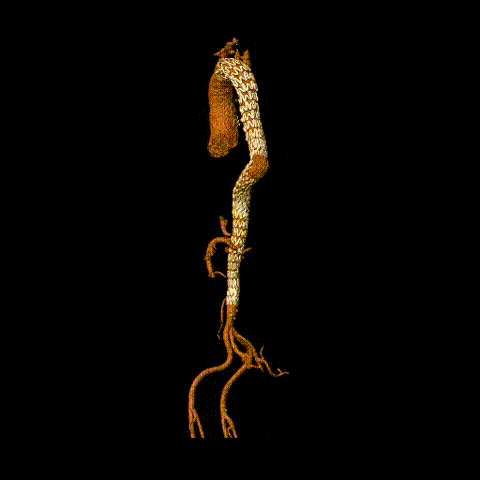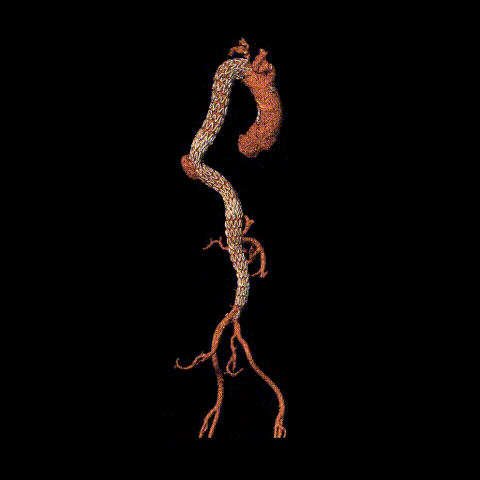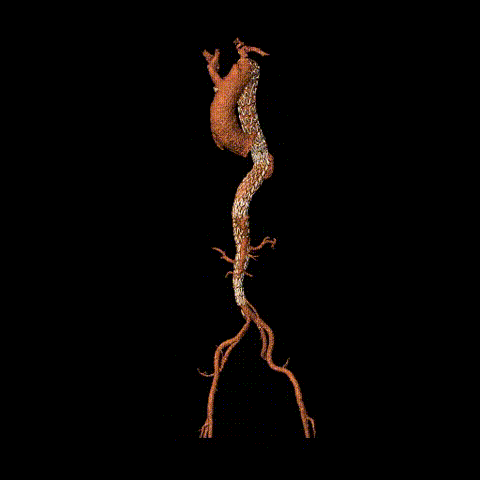
Postoperative CTA reconstruction
Summary
The Fabulous® Thoracic Aortic Stent System was designed and developed specifically for aortic coarctation and integrates the therapeutic concepts of the PETTICOAT technology. The design of the bare stent helps to widen the true lumen of the distal end of the coarctation and compress the false lumen.
Post-marketing clinical trials have initially demonstrated that the Fabulous® Thoracic Aortic Stent System promotes true lumen enlargement and false lumen narrowing, improves the rate of complete thrombosis of the false lumen without affecting the blood supply of the visceral branch arteries, and reduces the rate of paraplegia and lower limb ischemia compared to comparable products.
In patients with extensive involvement of the entrapment, where distal true lumen dilatation is found to be unsatisfactory after implantation and ischemia in the visceral area has not improved, a second or even a third bare stent may be renewed.
Introduction of Experts

Prof. Fu Weiguo PhD supervisor
Director, Department of Vascular Surgery, Zhongshan Hospital, Fudan University, Shanghai, China
Director, Institute of Vascular Surgery, Fudan University
Deputy Director of the National Clinical Research Center for Radiation and Therapeutics
Former Chairman of Shanghai Medical Association Vascular Surgery Branch
Chairman of the Vascular Surgery Branch of Shanghai Municipal Medical Association
Chairman of Vascular Surgery Branch of Cross-Strait Medical and Health Exchange Association (CSMHEA)
Vice Chairman of Vascular Surgery Group of Chinese Medical Association Surgery Branc
Vice Chairman of Vascular Surgery Branch, Chinese Physicians Association (CPA)
Endoluminal Angiology Committee of Chinese Physicians Association
Chairman of Aortic Clamping Specialist Committee
Vice Chairman, Endovascular Committee, Chinese Medical Doctors' Association, China
Vice Chairman, Vascular Surgery Committee, Gerontology Branch, Chinese Medical Doctors Association (CMA)
Vice Chairman, Large Vessel Intervention Committee, Interventionalists Branch, Chinese Medical Doctors Association
Vice Chairman, Vascular Surgery Committee, China Association for the Promotion of International Exchange in Healthcare (CAPIEH)
Editorial board member of Chinese Journal of Medicine, Chinese Journal of Surgery, Chinese Journal of Practical Surgery, etc.


