Branch reconstruction is currently the core problem faced by endovascular aortic repair, such as supra-arch branch reconstruction, visceral artery reconstruction and internal iliac artery reconstruction. On November 15, 2023, at the 50th International Congress of Vascular and Endovascular Diseases (VEITH 2023) held in New York, USA, Professor Guo Wei from the First Medical Center of Chinese PLA General Hospital shared the working principles and latest progress in clinical research of the new branch reconstruction devices WeFlow and G-Branch series products for juxtarenal AAA, TAAA and aortic arch repair.

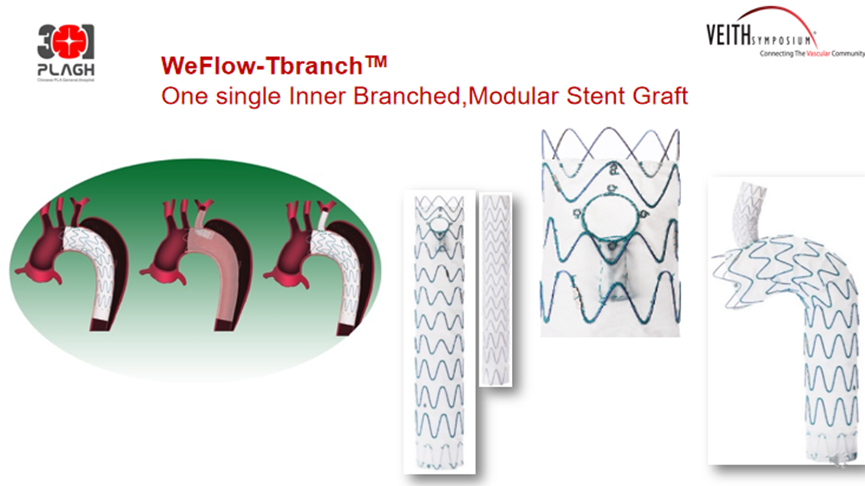
WeFlow-Tbranch™Single Inlay Thoracic Stent System
The WeFlow-Tbranch™Single Inlay Branch Thoracic Stent Graft System was originally designed with endovascular reconstruction of the left subclavian artery. It can be used to treat Stanford type B aortic dissection near the left subclavian artery and other aortic lesions.
The prospective, multicenter Guest study evaluated the safety and efficacy of this product in the treatment of Standord Type B dissection near the left subclavian artery. This study was a multicenter, single-arm study that included 120 patients. The mean distance from the proximal breach to the LCCA was 26.58 ± 6.90 mm. Primary safety endpoint: freedom from MAE at 30 days post-procedure was 95.83%. Of these, 1 died of any cause, 3 ischemic strokes, 2 respiratory failures, and 1 paraplegia.
Primary efficacy endpoint: Immediate postoperative technical success was 99.17% (119/120). One hundred and seven patients completed the 2-year follow-up without stent or branch migration, with a type I and type III endoleak rate of 2.30% and branch occlusion rate of 2.30% (Table 1).
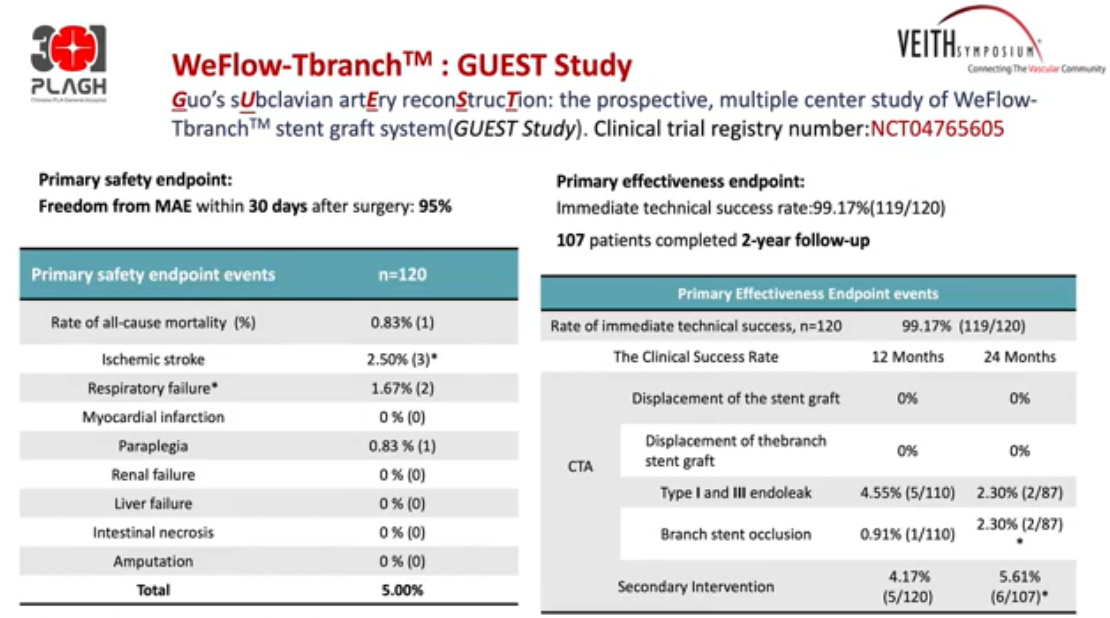
Table 1. Guest Study Safety and Efficacy Endpoints Events
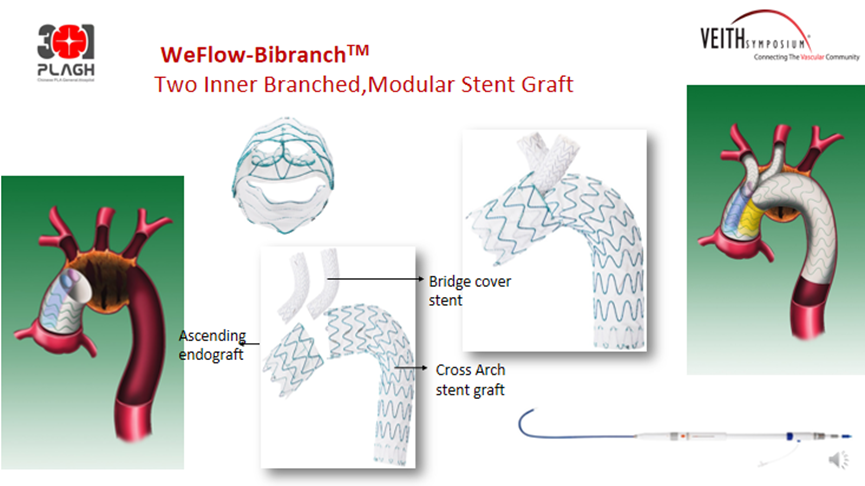
WeFlow-Bibranch™Dual Embedded Aortic Arch Stent Graft System
WeFlow-Bibranch™ uses a modular built-in design that achieves endovascular reconstruction IA and LCCA by combining ascending main, bridging, and transarch stent graft systems, ensuring that cerebral blood flow is not compromised during surgical procedures.
WeFlow-Bibranch™ First in Man (FIM) CTA Follow-up Results: Fifteen patients, ages 53 to 76 years, were enrolled. There were 10 true aneurysms, 2 PAU, 2 dissection, and 1 pseudoaneurysm. Seven patients underwent combined LCCA-LSA bypass surgery. All procedures were immediately successful. Postoperatively, 1 stroke occurred and 1 died of cerebral hemorrhage. There were no endoleaks, no branch occlusions, and no stent dislodgments during follow-up.
Preliminary results from the prospective, multicenter clinical study, GIANT study, demonstrate the safety and efficacy of WeFlow-Bibranch ™. A total of 80 patients were enrolled in the study involving 15 clinical centers. The primary end point was 30-day all-cause mortality 3.80%, stroke 7.59%, and 6-month stroke 9.09%; secondary end points were technical success 100%, 6-month freedom from type I/III endoleaks, migration, or reintervention, and branch patency 100% (Table 2).
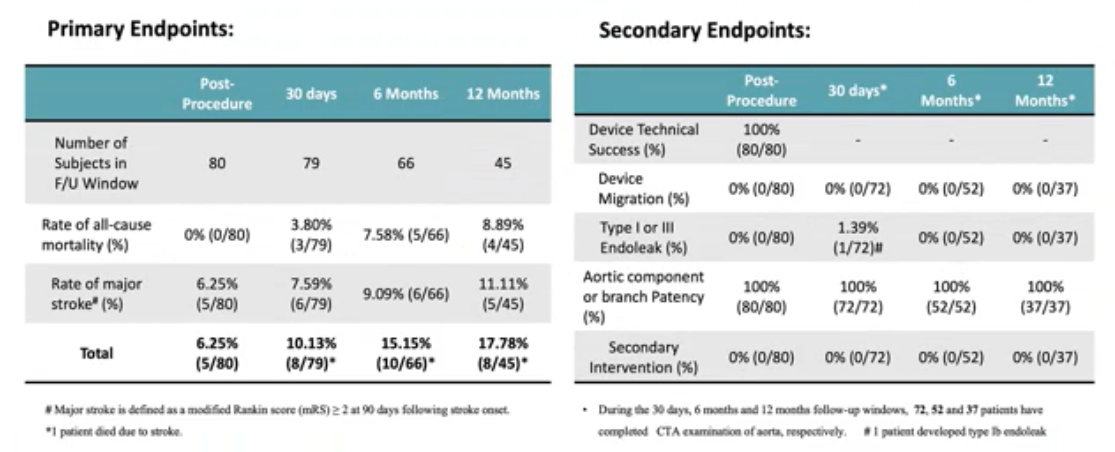
Table 2. GIANT Study Primary and Secondary Endpoints
WeFlow-Tribranch™ Module Embedded Aortic Arch Triple Branch Stent Graft System
WeFlow-Tribranch™ achieves true total endoluminal reconstruction of three branches on the aortic arch. The FIM study has now been conducted: 17 patients were enrolled. 30-day MAE results: 3 all-cause deaths, 1 stroke, 1 respiratory failure, 1 paraplegia.
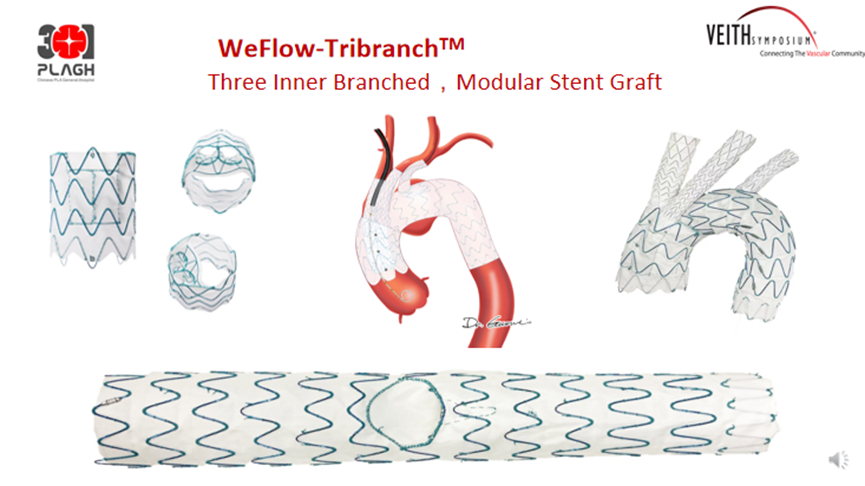
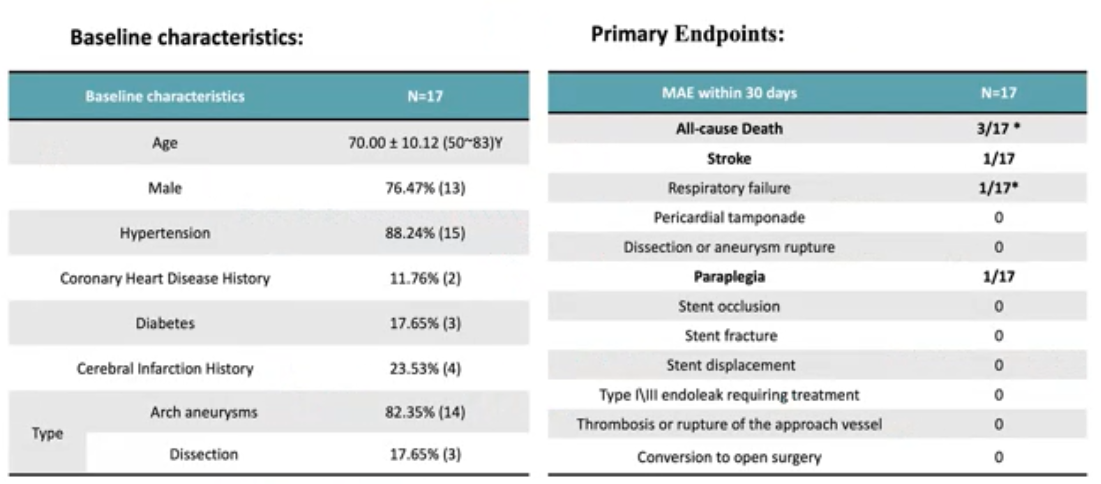
表3. FIM研究结果
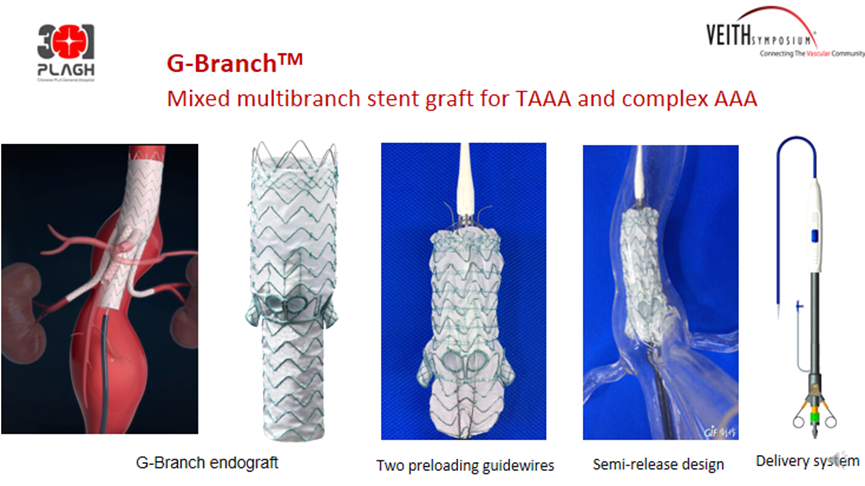
G-Branch™ Thoracoabdominal Aortic Stent Graft System
The G-Branch ™ Thoracoabdominal Aortic Stent Graft System consists of two inlay and two everted branches for reconstruction of the four branches of TAAA/PAAA.
The product showed excellent patency rates and endoleak prevention in the FIM study. The national multicenter clinical study GUARANTEE was initiated on 12 November 2021 and is expected to end on 31 December 2028 to assess the safety and efficacy of the product in the treatment of thoracoabdominal aortic aneurysm (TAAA) and perirenal abdominal aortic aneurysm (PAAA), with 73 patients enrolled at 15 centers. The technical success rate was 95.89%, with one type IA and one type III endoleak and one left renal artery occlusion (Table 4). Seventy-three patients completed the 30-day follow-up, 1 acute myocardial infarction, 1 acute cerebral infarction, and 2 paraplegia. Sixty-three patients completed the 6-month follow-up with an all-cause mortality rate of 0%. Forty-eight patients completed the 12-month follow-up, with an all-cause mortality rate of 2.08%, a type I/III endoleak rate of 4.17%, and 97% renal artery patency (Table 5).
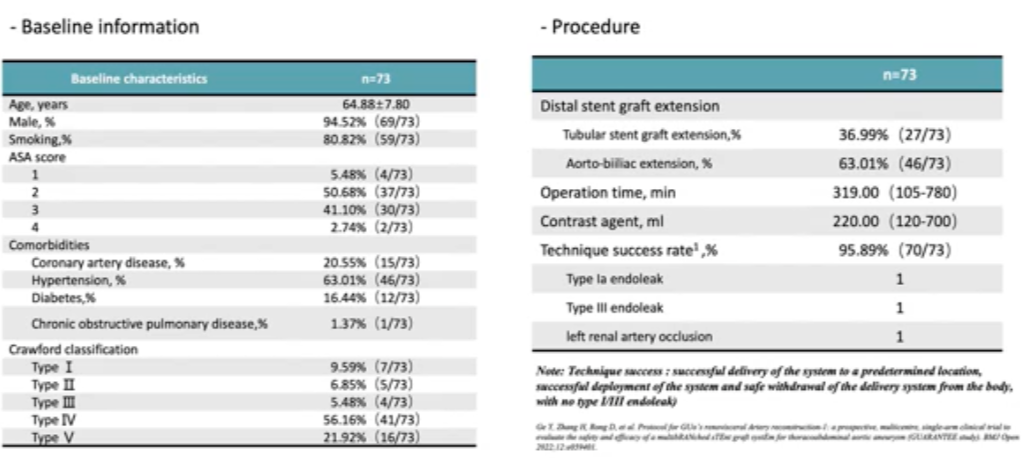
Table 4. Baseline and procedural results of the GUARANTEE study
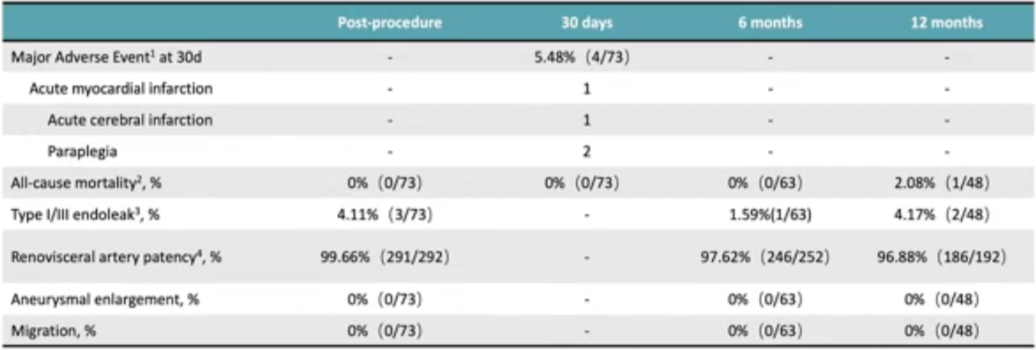
Table 5. Follow-up Results of GUARANTEE Study
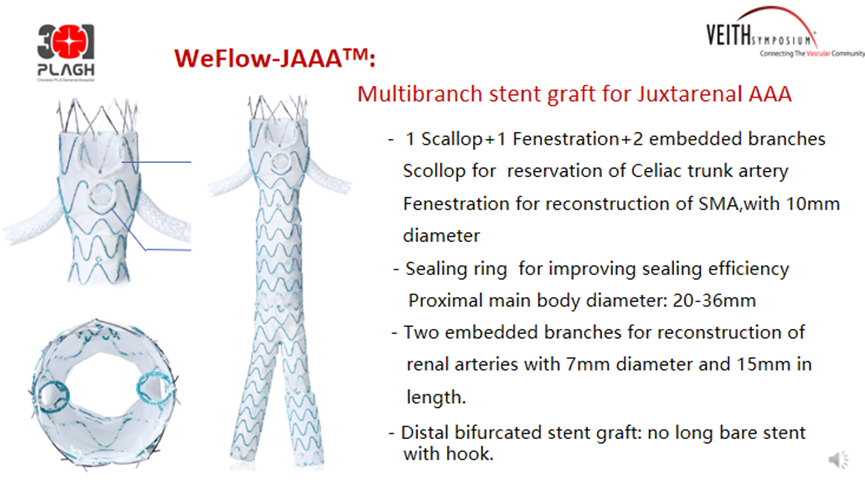
WeFlow-JAAA™ Multi-Branch Abdominal Aortic Stent System
The WeFlow-JAAA™ AAA Stent System is designed primarily for the treatment of juxtarenal abdominal aortic aneurysms. The structure includes one notch, one fenestra and two embedded branches for the reconstruction of CA, SMA and RA, respectively. Slots and window edges have sealing rings designed to prevent endoleak.
The FIM study for this product showed no Type I and Type III endoleaks at 90-day follow-up. On this basis, Professor Guo Wei 's team carried out a national multicenter clinical study, the GREAT study, in order to evaluate the efficacy of this product in the treatment of J-AAA, and plans to include 106 patients in 20 centers in China. The GREAT study was officially launched on 23 February 2022, and 106 patients have been enrolled and completed the procedure so far. Inclusion criteria Aneurysm to SMA length > = 4 mm and landing zone angulation < 60 °. Primary safety endpoint: 1 patient died within 30 days. Primary efficacy endpoint: Immediate postoperative technical success rate 98.82%. Thirty patients completed the 6-month follow-up without endoleak, 96.7% had patent target vessels, and the reintervention rate was 9.6%. Twelve patients completed the 12-month follow-up without endoleak and 100% of target vessels were patent without reintervention (Table 6).
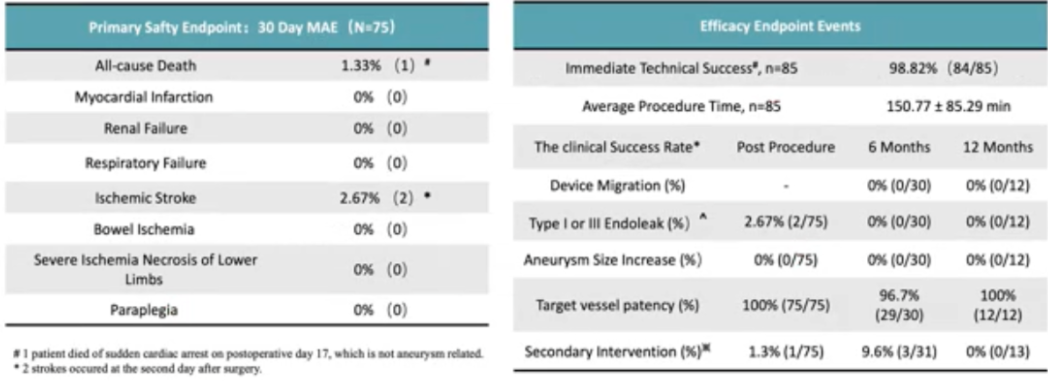
Table 6. GREAT Study Safety and Efficacy Endpoints
Summary
WeFlow-Tbranch ™ is designed for reconstruction of LSA and preliminary results at 1-year follow-up demonstrate its safety and efficacy. WeFlow-BiBranch ™ is designed specifically for reconstruction of IA and LCCA and can be combined with LCCA-LSA bypass surgery if necessary. WeFlow-TriBranch ™ is designed for total endoluminal aortic arch replacement. G-Branch ™ is designed for reconstruction of four branch vessels of TAAA/PAAA. WeFlow-JAAA ™ is designed for the reconstruction of the renal arteries and SMA to address the endovascular treatment of JAAA. In the future, more cases and longer follow-up periods are needed to provide more evidence for each product.
Article Source:Clinic門诊腔内血管


