Author: Dr. Michael Piorkowski
Institution: Bethanien Cardiovascular Center, Frankfurt, Germany
Abstract
This presentation focuses on the application of the ClotTriever device for treating deep vein thrombosis (DVT), emphasizing the effectiveness of mechanical thrombectomy without thrombolysis. Data from the Clout registry demonstrate that the ClotTriever device achieves significant clot removal in over 90% of cases, while eliminating the need for thrombolytic drugs and reducing the risk of complications.
Introduction
Deep vein thrombosis (DVT) is a leading cause of pulmonary embolism and post-thrombotic syndrome (PTS). While traditional thrombolysis is effective, it carries the risk of bleeding and longer ICU stays. The ClotTriever mechanical thrombectomy system is designed to remove clots without the use of thrombolytic drugs, minimizing the risks of bleeding and other complications.
ClotTriever System Overview
• Components: The ClotTriever system includes a catheter, sheath, and thrombectomy collection bag, allowing for mechanical removal of clots without thrombolysis.
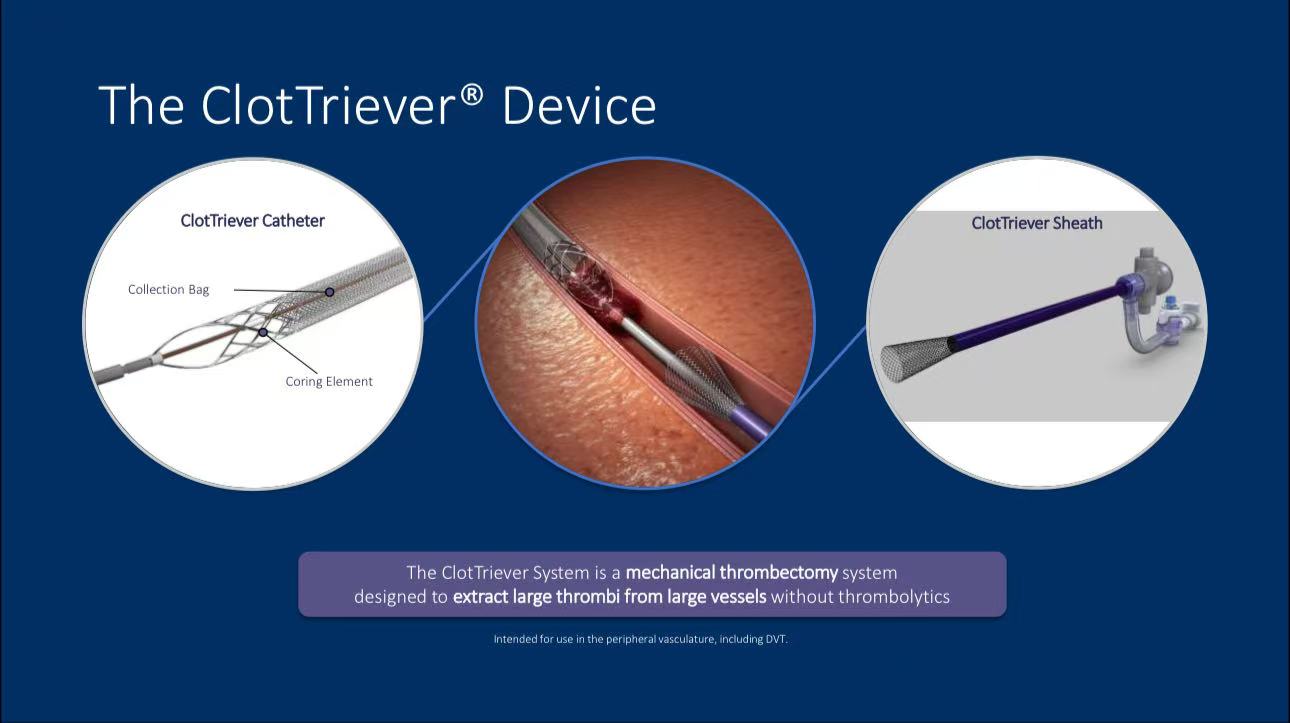
• Indications: The device is primarily used for lower extremity DVT, especially in proximal cases.
Clout Registry Results
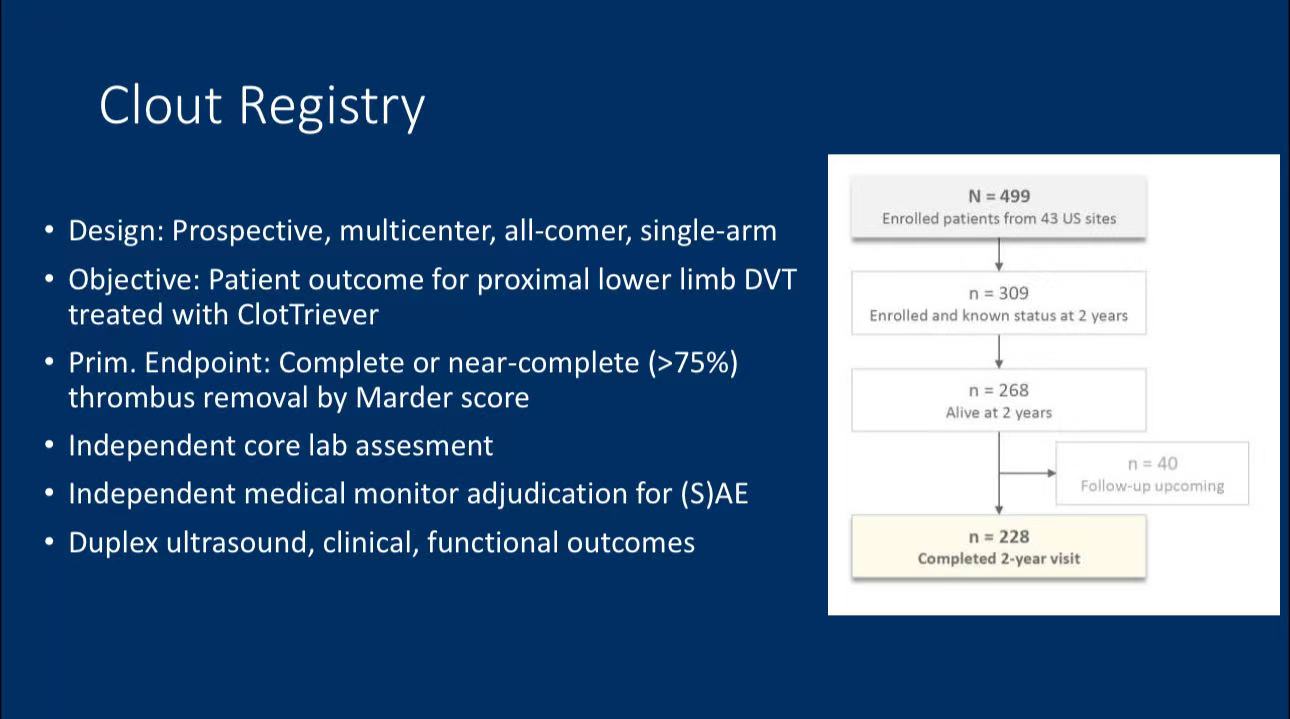
• Study Design: The Clout registry is a prospective, multicenter, single-arm study designed to evaluate the efficacy of the ClotTriever device in treating proximal lower limb DVT patients.
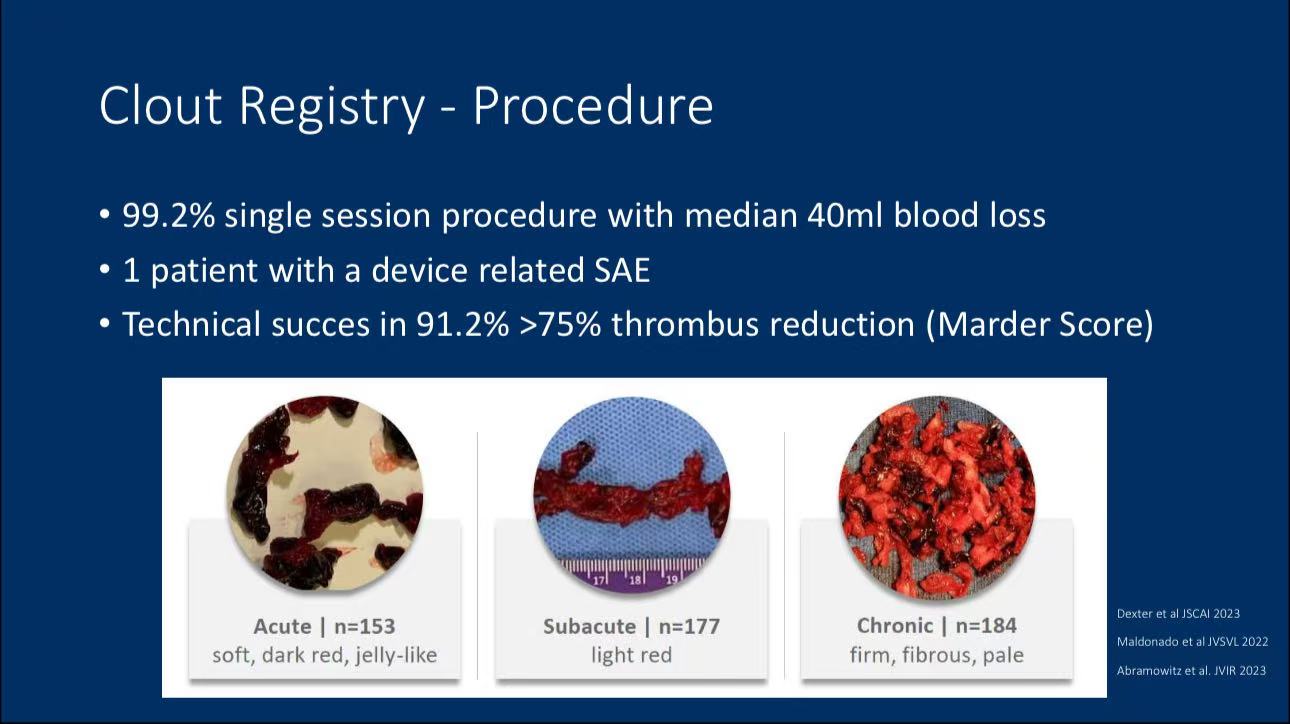
• Primary Endpoint: The main goal was to achieve a clot removal rate of over 75% based on the Marder score. The results showed that 91.2% of patients met this standard, with 99.2% completing treatment in a single session. The average blood loss was 40 mL.
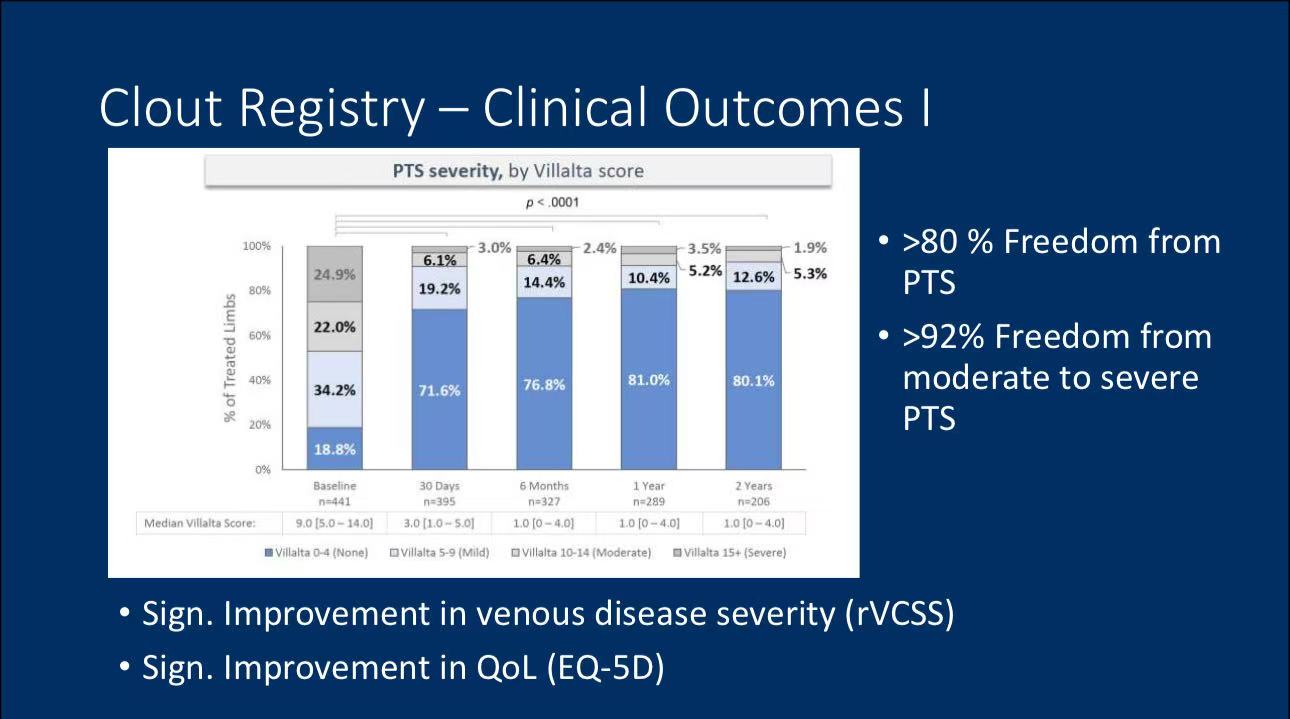
• Clinical Outcomes: Two-year follow-up data indicate that the ClotTriever device performed well in preventing moderate-to-severe PTS, with over 90% of patients free from severe PTS and a rethrombosis rate of less than 8%.
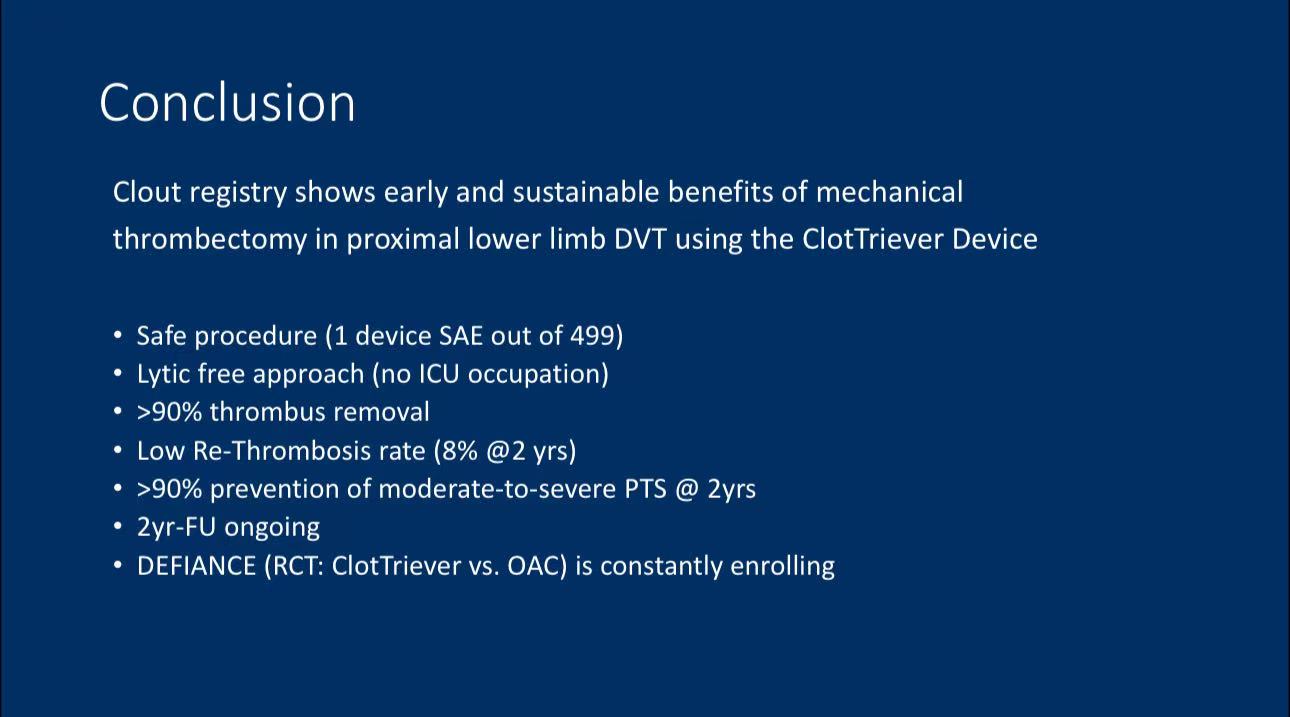
Conclusion
1. The ClotTriever device demonstrated significant efficacy in mechanical thrombectomy for DVT, with a technical success rate of 91.2% and a reduction in complications associated with thrombolysis.
2. The device performed well in reducing rethrombosis and preventing moderate-to-severe PTS, making it particularly suitable for proximal lower limb DVT patients.
3. The Clout registry provides robust evidence supporting the ClotTriever device as a valuable tool in DVT treatment, with further studies expected to explore its use in other venous pathologies.
Contact Us
For submissions, please contact us at: endovascluar@simtomax.cn
Thank you for your attention, and let’s continue to safeguard health together!
More international information available at:
• Facebook: Vasco Knight
• Instagram: knight_vasco


