Author: Dr. Michael Lichtenberg
Institution: Vascular Center Arnsberg, Germany
Abstract
This study presents the 24-month clinical follow-up results of the Aspirex Mechanical Thrombectomy System for treating acute venous occlusions, with a focus on technical success, procedural success, and long-term patency. The findings demonstrate that the Aspirex system achieves high technical success and favorable patency rates, making it an effective treatment option for acute iliac-femoral deep vein thrombosis (DVT).
Study Design
•Design: The P-MAX study is a prospective, multicenter, post-market observational study aimed at evaluating the efficacy of the Aspirex system in treating acute venous occlusions. The study primarily focuses on patients with iliac-femoral DVT, including some cases of dialysis access occlusions.

•Objective: The main goal is to assess the procedural and technical success of the Aspirex system over 24 months, while analyzing primary vessel patency.

Key Results
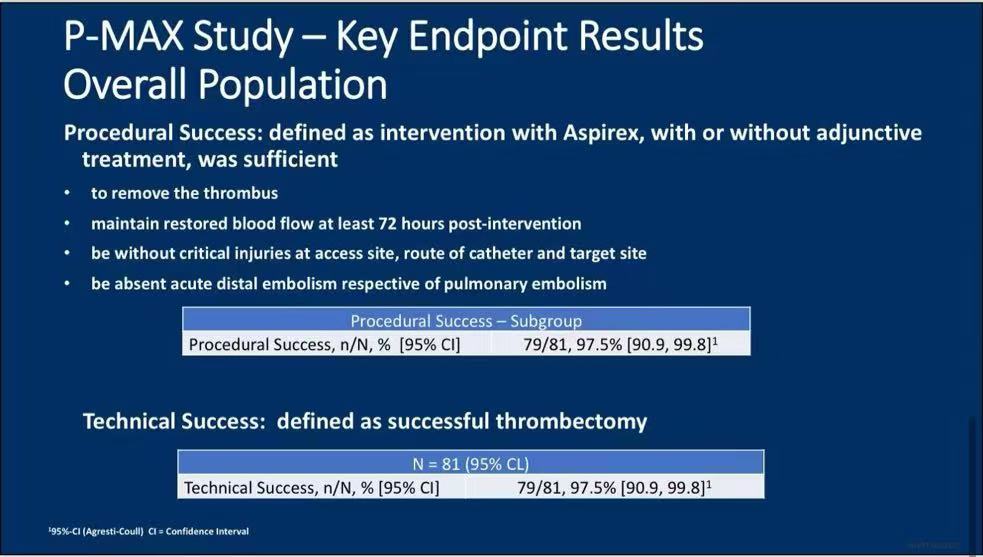
1.Technical Success: Out of 81 patients, the Aspirex system achieved a 97.5% technical success rate, showcasing its efficiency in clot removal .

2.Procedural Success: The procedural success rate was also 97.5%, defined by the successful removal of thrombi and restoration of blood flow for at least 72 hours post-operation without severe complications.
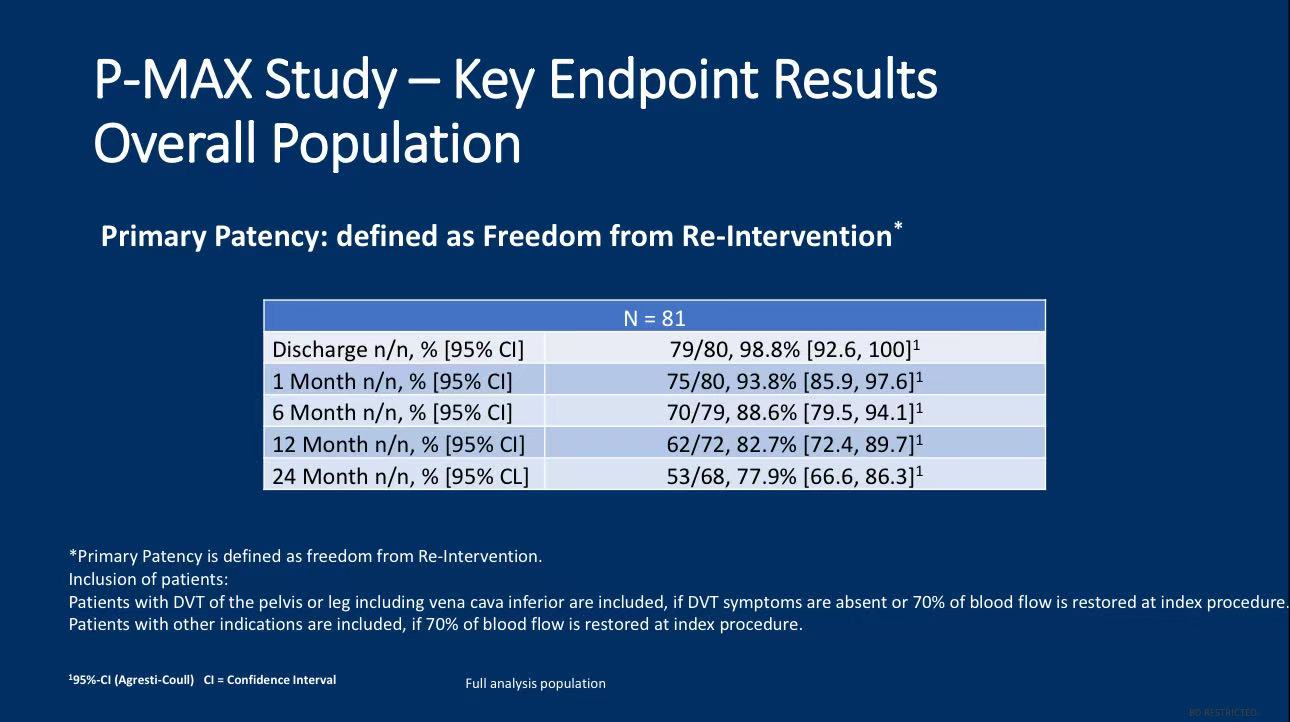
3.Patency Rate: The primary patency rate at 24 months was 77.9%, meaning most patients did not require further revascularization procedures during this period.
Safety Results
•Serious Adverse Events (SAE): During the 24-month follow-up, 59.7% of patients reported serious adverse events, though only 3.1% were device-related.

Conclusion
1.The Aspirex Mechanical Thrombectomy System demonstrates high technical success and favorable long-term patency in treating acute venous occlusions, making it an effective and safe treatment option for patients with acute DVT.
2. Although there was a notable incidence of serious adverse events, most were unrelated to the device, supporting the potential of the system in clinical practice.
3. Long-term follow-up data suggest that the Aspirex system reduces the need for reintervention, enhancing patient quality of life.
Contact Us
For submissions, please contact us at: endovascluar@simtomax.cn
Thank you for your attention, and let’s continue to safeguard health together!
More international information available at:
•Facebook: Vasco Knight
•Instagram: knight_vasco


