Author: Dr. Ioannis Passaloglou
Institution: Department of Vascular and Endovascular Surgery, St. Gertrauden Hospital, Germany
Abstract
Dr. Ioannis Passaloglou presented the latest updates from the CERAB registry trial, assessing the safety and efficacy of the BeGraft aortic and peripheral stent systems in the treatment of iliac occlusive disease. The trial is a multicenter, non-randomized clinical study, designed to gather data over a 48-month follow-up period, aiming to validate the clinical performance of CERAB technology in the treatment of this condition.
Study Design
• Study Locations: The trial is being conducted across 15 clinical centers in Germany and 3 centers in the Netherlands.
• Duration: The study spans 48 months, including a 24-month enrollment phase and a 24-month follow-up period.
• Participants: A total of 109 patients have been enrolled, with recruitment starting on May 4, 2024.

Primary Endpoints
• Target Lesion Revascularization (TLR): Clinically driven TLR within 12 months.
• Freedom from Repeat Revascularization.
• Device and procedure-related Serious Adverse Device Events (SADE) and Serious Adverse Events (SAE) rates.

Secondary Endpoints
• Technical Success: The rate of successful procedures.
• Risk of Conversion to Open Surgery.
• Patency Rates: Including primary patency, primary-assisted patency, and secondary patency rates.
• Patient-reported Outcomes: Improvements in the Walking Impairment Questionnaire (WIQ), Quality of Life Questionnaire, and Ankle-Brachial Index (ABI) .

Safety Endpoints
• SADE and SAE Occurrence: Within 1 month, 6 months, and 24 months post-procedure.
• Mortality and Survival Rates: Within 30 days post-procedure.
• Major Adverse Events (MAE): Occurrence within 30 days, 6 months, 12 months, and 24 months post-procedure.

Clinical Cases and Conclusion
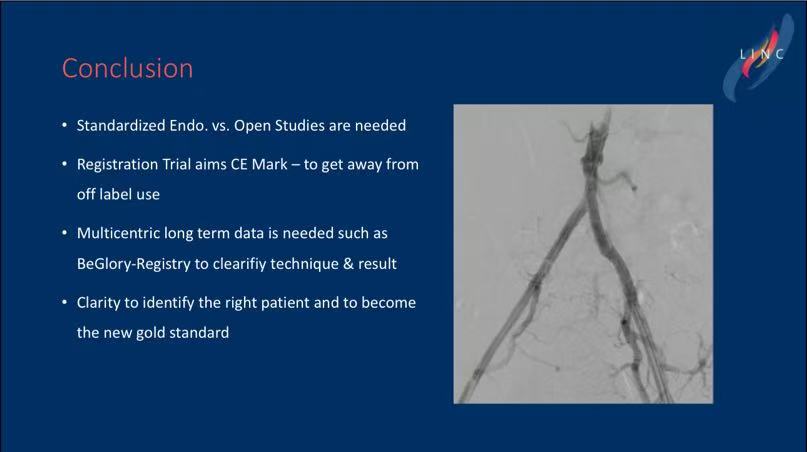
• Imaging Criteria: Patients must meet the CERAB treatment criteria, with target lesions located at least 1 cm below the renal arteries, avoiding the need for chimney techniques.
• Conclusion: Comparative studies between standardized endovascular treatments and open surgery require more data. The registry trial aims to support the BeGraft stent system’s bid for CE marking, with long-term multicenter follow-up data needed to confirm the technology’s efficacy .
Contact Us
For submissions, please contact us at: endovascluar@simtomax.cn
Thank you for your attention, and let’s continue to safeguard health together!
More international information available at:
• Facebook: Vasco Knight
• Instagram: knight_vasco


