Author: Prof. Dr. Francesco Liistro
Institution: Head of Cardiovascular Interventions, San Donato Hospital, Arezzo, Italy
Summary
The DEBATE BTK DUELL trial compares the efficacy and safety of Paclitaxel and Sirolimus drug-coated balloons (DCBs) in treating below-the-knee (BTK) artery disease. The study primarily evaluates differences in late luminal loss (LLL), target lesion revascularization (TLR), and major adverse events (MAE) between these two DCBs. Initial results demonstrate favorable outcomes for both devices in preventing restenosis, with a slight patency advantage for Paclitaxel-coated devices.
Key Points
1.Study Design:
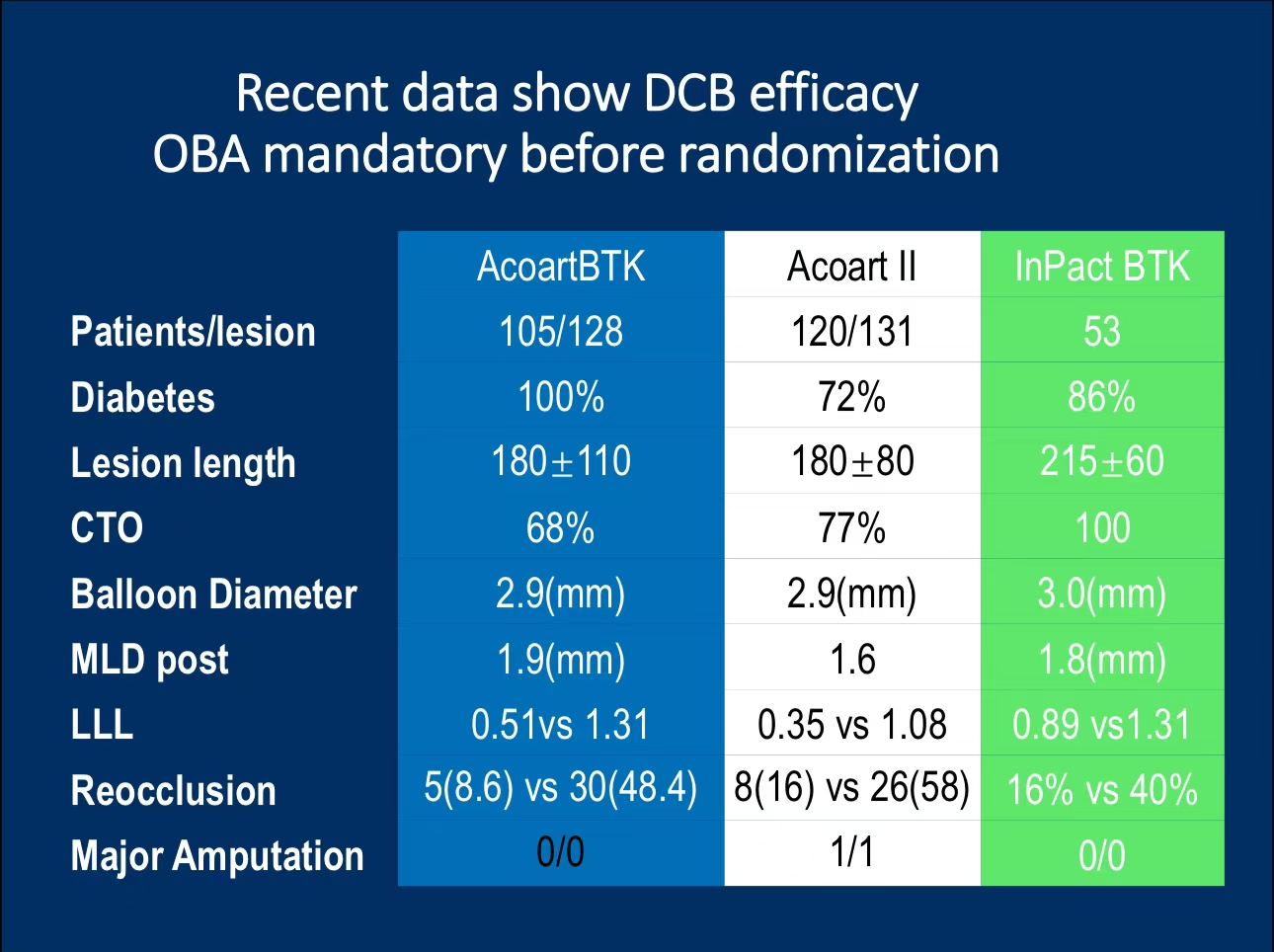
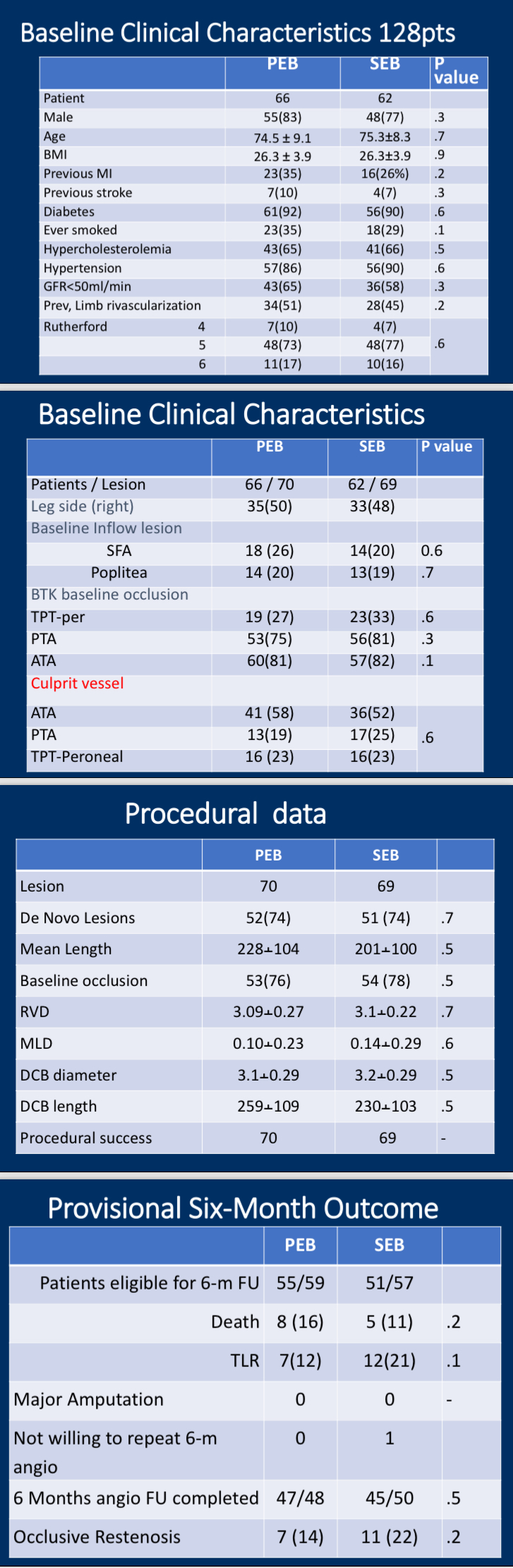
•The trial included 128 patients with BTK stenosis, 92% of whom were diabetic. Patients were randomly assigned to receive either Paclitaxel or Sirolimus DCB treatments.
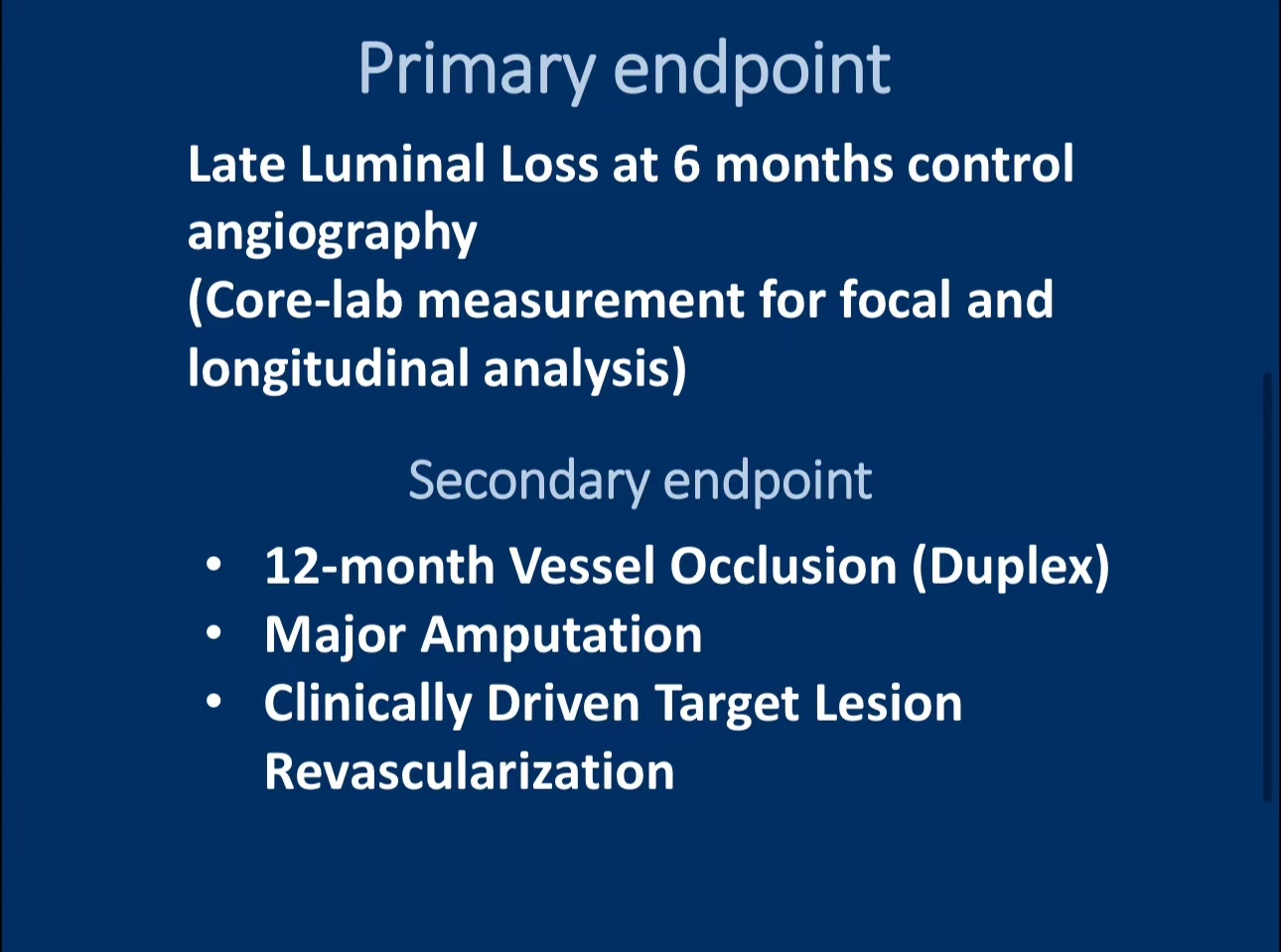
•The primary endpoint was late luminal loss at 6 months, with secondary endpoints including TLR, major amputations, and overall safety at 12 months.
2.Clinical Outcomes:
•Restenosis and TLR: After 6 months, the Paclitaxel group showed a 14% restenosis rate, compared to 22% for Sirolimus. TLR rates were also lower for Paclitaxel at 12%, versus 21% for Sirolimus.
•Major Adverse Events: Neither group experienced significant amputations, though the Paclitaxel group had a slightly higher mortality rate (16% vs. 11%), with no statistically significant difference.
3.Case Studies:
•Case 1: A 66-year-old male with diabetes and BTK stenosis treated with Paclitaxel showed no restenosis at 6 months, with improved walking capacity.
•Case 2: A 62-year-old female with diabetes and chronic limb ischemia (CLI) treated with Sirolimus experienced restenosis at 6 months but recovered after successful balloon angioplasty.
Conclusion
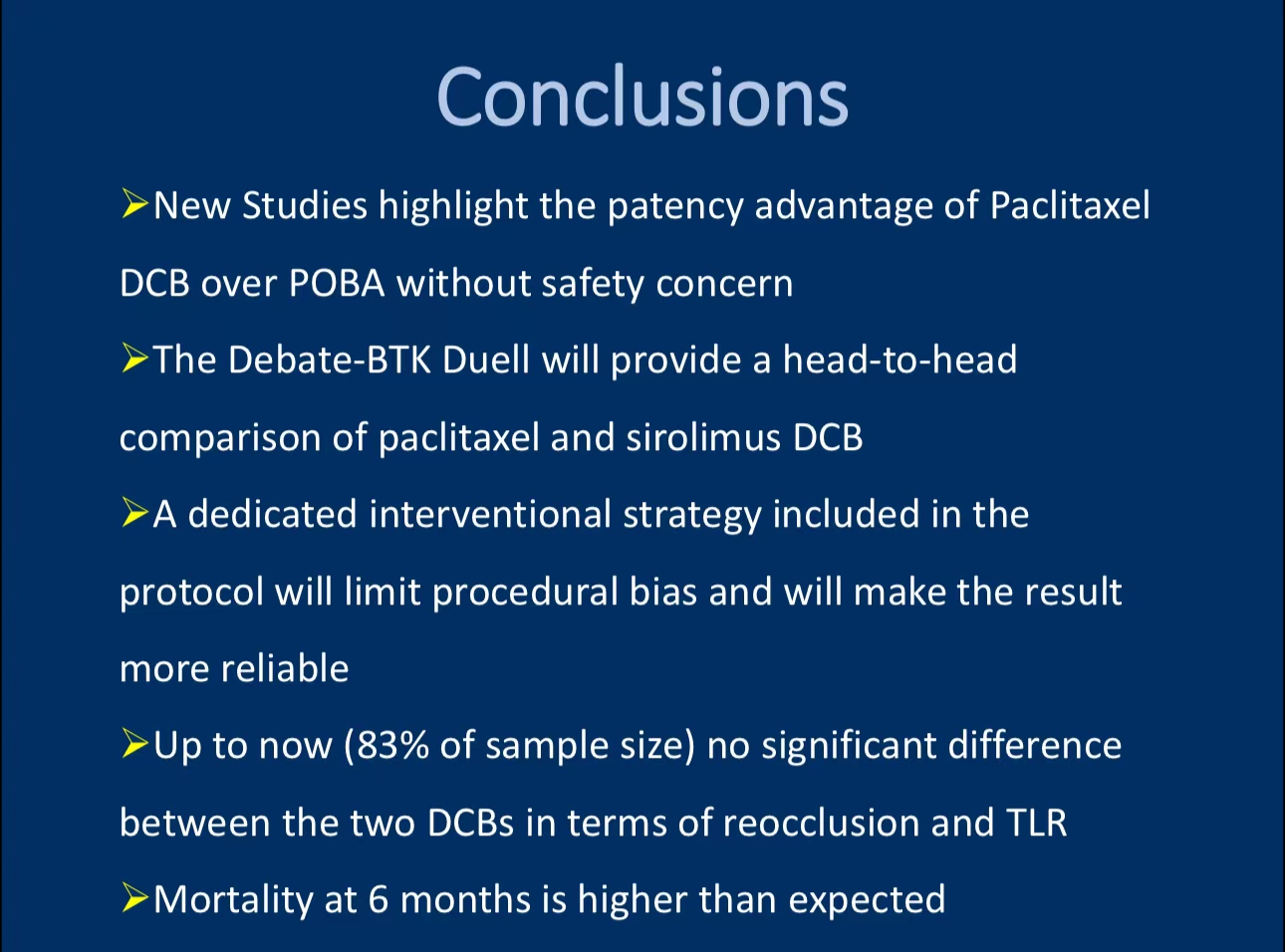
1. Both Paclitaxel and Sirolimus DCBs demonstrate strong efficacy in treating BTK disease, with Paclitaxel showing a slight advantage in patency rates.
2. Sirolimus DCBs performed well in treating long lesions.
3. Although the Paclitaxel group had a higher mortality rate, both devices showed overall safe profiles.
Contact Us
For submissions, please contact us at: endovascluar@simtomax.cn
More international information available at:
•Facebook: Vasco Knight
•Instagram: knight_vasco
Let’s continue safeguarding health and showcasing your expertise globally!


