Author: Prof. Dr. Stefano Barco
Institution: Vascular Department, University of Zurich, Switzerland
Summary
This report updates the progress of the SirPAD trial, particularly focusing on the application of Sirolimus-coated balloons (SCB) in treating femoropopliteal and below-the-knee peripheral arterial disease. The primary objective of the trial is to compare the effectiveness of SCB against plain old balloon angioplasty (POBA), with a focus on major adverse limb events (MALE) occurring within one year.
Introduction
The SirPAD trial evaluates the safety and efficacy of SCBs in peripheral artery disease (PAD). Recent studies have shown the potential of drug-coated balloons in reducing restenosis. This report provides mid-term analysis results of the SirPAD trial, specifically examining femoropopliteal and below-the-knee MALE, including unplanned major amputations and target lesion revascularization.
Study Design
• Primary Objective: The trial aims to evaluate the non-inferiority of SCBs in suppressing target lesion restenosis and reducing MALE rates compared to standard POBA within one year .

•Participants:Patients with femoropopliteal and below-the-knee stenosis were randomized to receive SCB or POBA during angioplasty .
Key Findings
• MALE: The one-year MALE rate in the SCB group was 10%, slightly higher than initially projected but without concerning mortality or safety signals.

• Patient Demographics: 64% of patients were male, with an average age of 75. Acute limb ischemia was present in 13% of patients, and 33% had critical limb ischemia (CLI).
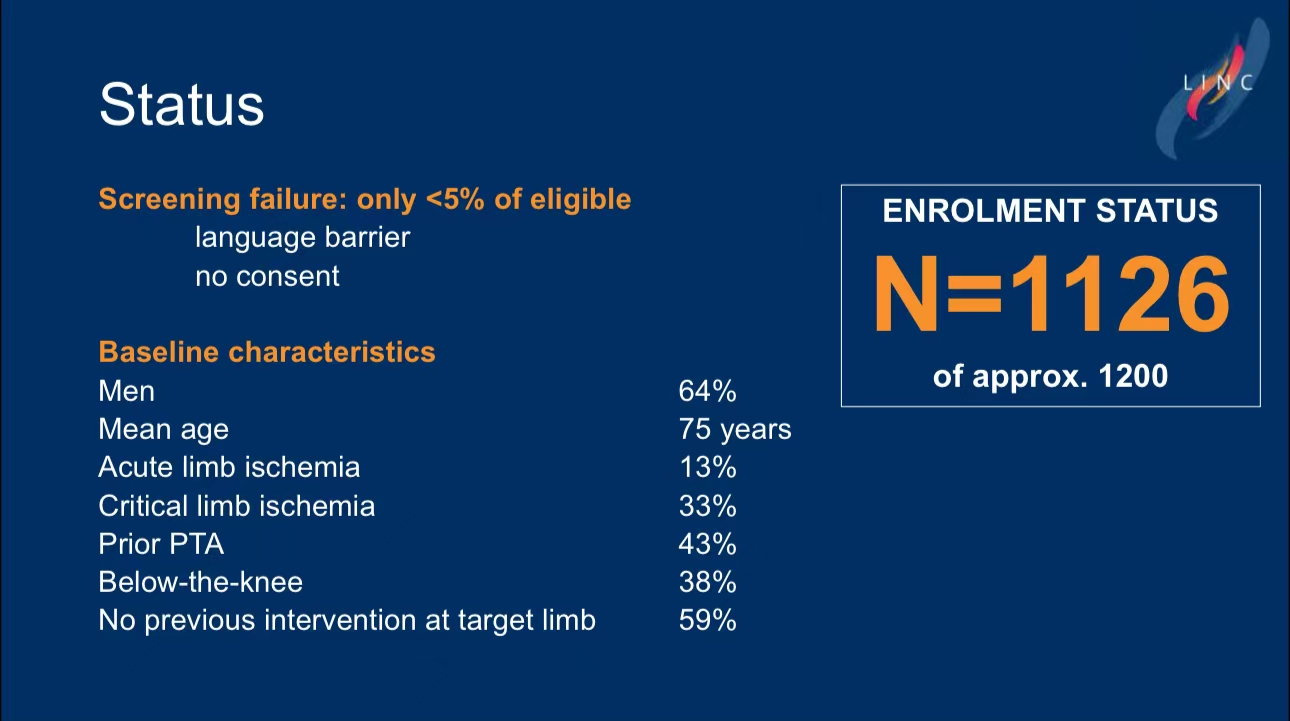
• Mid-term Results: As of March 2024, 1126 patients have been enrolled. Preliminary data show SCB as highly effective in restenosis suppression, with no significant difference in overall mortality between the two groups.

Case Studies
• Case 1: A 65-year-old male with femoropopliteal stenosis underwent SCB treatment and showed significant improvement with no target lesion revascularization after one year.
• Case 2: A 72-year-old female with CLI underwent SCB treatment. After six months, no restenosis or target lesion revascularization occurred, and her walking ability significantly improved.
Conclusion
1.The SirPAD trial shows promising mid-term results, with SCB demonstrating effectiveness and safety in treating femoropopliteal and below-the-knee arterial disease. Although the MALE rate is slightly higher than expected, the overall safety profile remains reassuring.
2.SCB presents potential advantages over traditional POBA in reducing restenosis and target lesion revascularization rates.
3.Further research will focus on long-term follow-up data to confirm the efficacy and safety of SCBs in PAD treatment.
Contact Us
For submissions, please contact us at: endovascluar@simtomax.cn
Thank you for your attention, and let’s continue to safeguard health together!
More international information available at:
• Facebook: Vasco Knight
• Instagram: knight_vasco


