Author: Dr. Sahil A. Parikh
Institution: Columbia University Vagelos College of Physicians and Surgeons, NewYork-Presbyterian/Columbia University Irving Medical Center
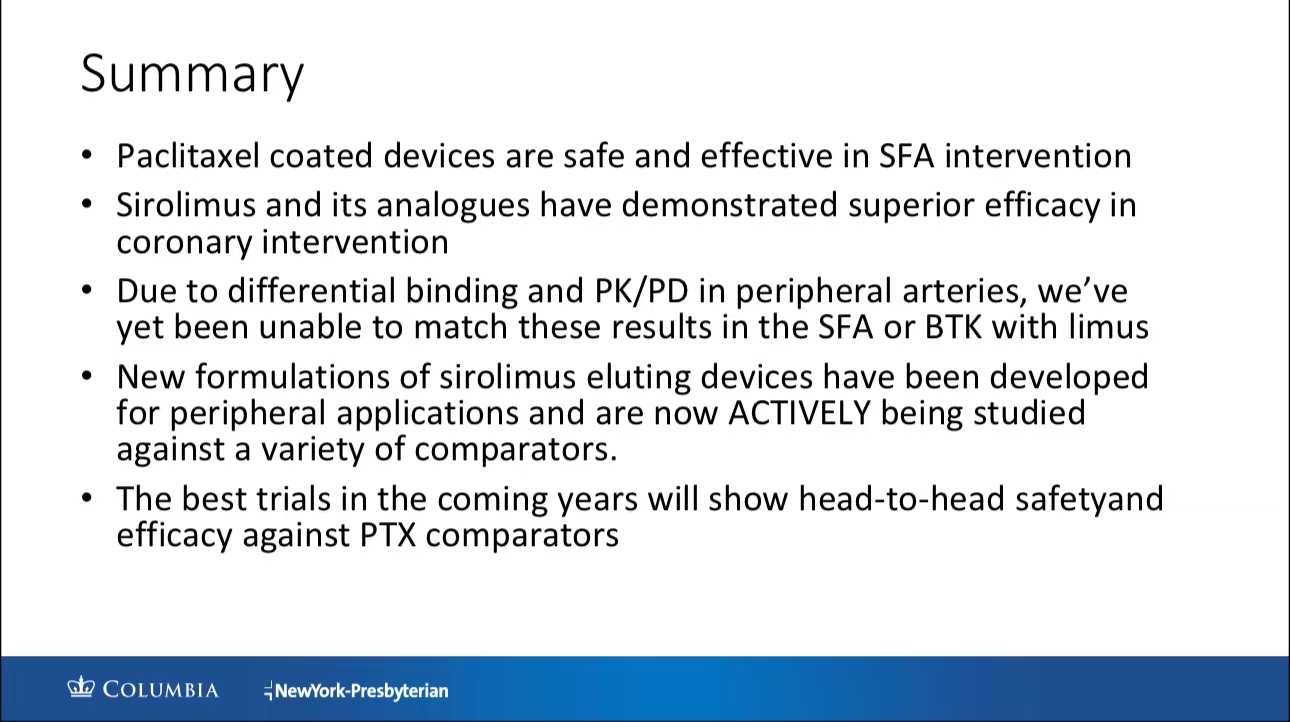
Summary
Dr. Sahil A. Parikh presented the current applications of drug-coated balloons (DCB) in treating peripheral arterial disease (PAD), with a focus on their comparison to other technologies such as drug-eluting stents (DES) and plain balloon angioplasty (PTA). The presentation highlighted clinical data on the safety and effectiveness of DCBs, particularly examining the risks of paclitaxel-based devices in long-term applications. While DCBs have demonstrated efficacy in several areas, controversies surrounding their safety, especially regarding mortality risks, continue to be debated.
Introduction
Drug-coated balloons (DCB) have become an essential tool in the treatment of PAD, particularly for preventing restenosis. These balloons are coated with drugs like paclitaxel or sirolimus to inhibit neointimal hyperplasia. Despite their benefits, concerns about the long-term safety of paclitaxel-based devices, especially regarding mortality, remain an ongoing discussion in the vascular community.
Treatment Options for SFA
•Conventional Options: For treating superficial femoral artery (SFA) disease, options include PTA, nitinol stents, DES, DCB, and other adjunctive technologies like cutting balloons and atherectomy.
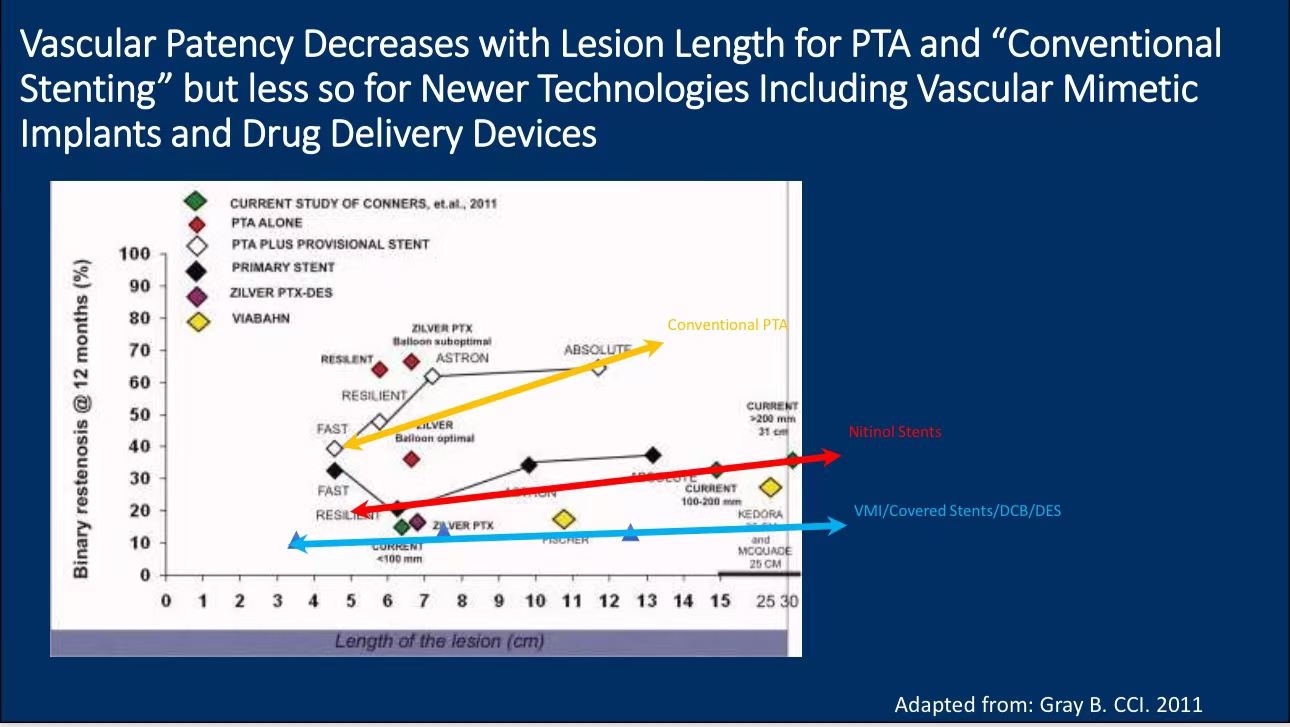
•Technological Comparison: Studies comparing DCB/DES with traditional stents have shown that while plain PTA has lower patency rates, it remains a viable option in certain cases. However, DCBs and DES have demonstrated superior outcomes in inhibiting restenosis, especially with newer stent designs.
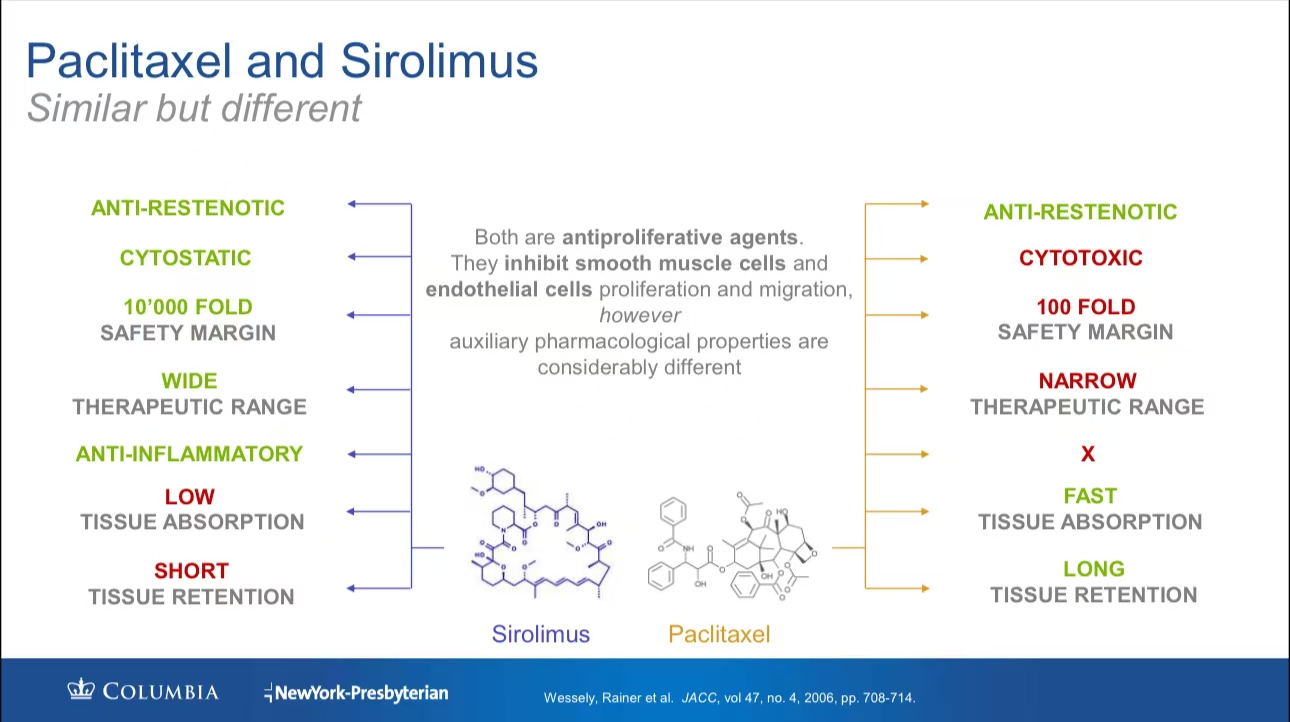
Comparison Between Paclitaxel and Sirolimus
•Drug Properties: Paclitaxel and sirolimus are both antiproliferative agents, but they differ significantly in pharmacokinetics. Paclitaxel is rapidly absorbed and retained in tissues for extended periods, whereas sirolimus offers broader therapeutic coverage with additional anti-inflammatory properties.
•Long-Term Safety Concerns: Recent debates have centered on the potential link between paclitaxel devices and increased mortality risk. Although most studies do not show clear mortality signals, some case reports suggest delayed vessel healing and tissue necrosis associated with paclitaxel (PPT 12).

Clinical Study Outcomes
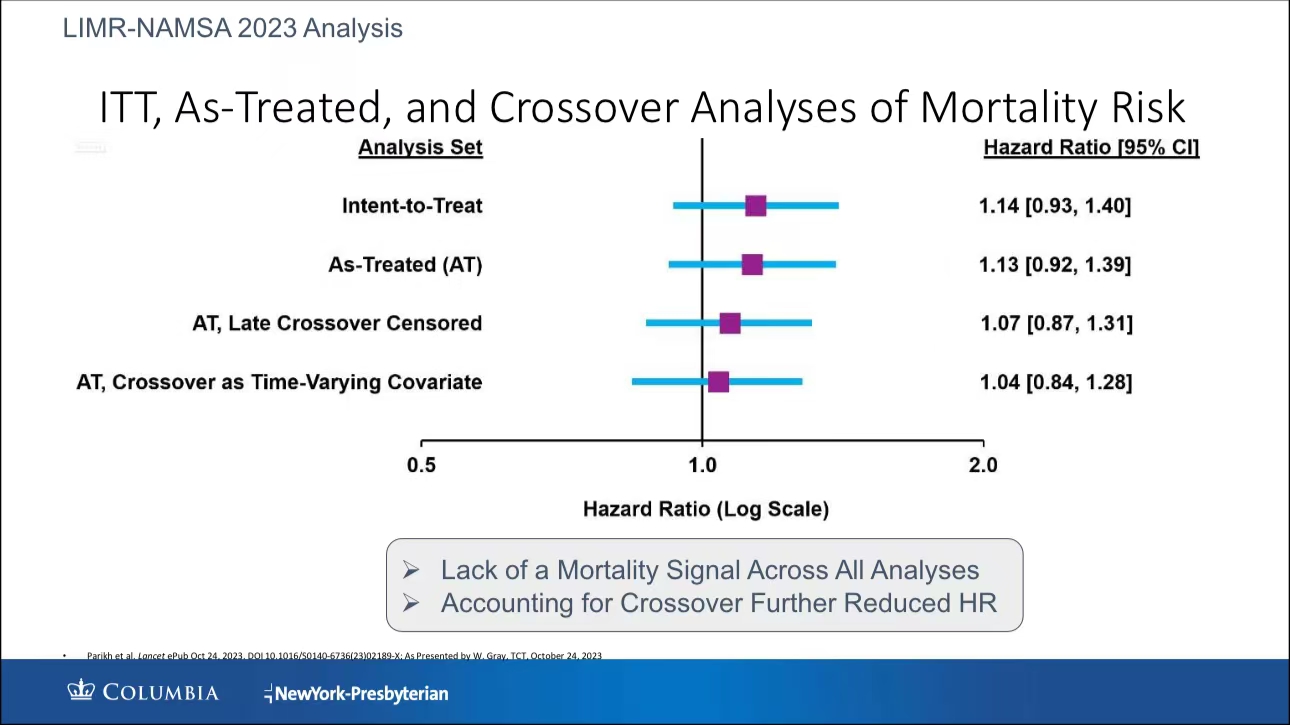
•LIMR-NAMSA 2023 Analysis: Parikh’s recent study, which included a cross-group analysis, showed minimal differences in mortality risks between paclitaxel and non-paclitaxel devices. As more data becomes available, the concern over mortality signals continues to diminish, suggesting a higher level of safety for paclitaxel devices in long-term use (PPT 15).
•MAGICAL BTK & SFA Trials: Dr. Parikh’s team is leading a randomized trial to evaluate sirolimus-coated balloons (SCB) in femoropopliteal and below-the-knee arteries. Preliminary results indicate good patency rates and safety for SCBs, although further research is required to compare SCBs directly with paclitaxel-based devices.

Conclusion
Drug-coated balloons have shown significant promise in treating peripheral arterial disease, particularly in reducing restenosis. While the long-term safety of paclitaxel-based devices has been a concern, recent research suggests a weaker link between these devices and mortality. Future studies will continue to compare the efficacy and safety of sirolimus versus paclitaxel devices, providing clearer guidance for clinical practice.
Contact Us
For submissions, please contact us at: endovascluar@simtomax.cn
Thank you for your attention, and let’s continue to safeguard health together!
More international information available at:
•Facebook: Vasco Knight
•Instagram: knight_vasco


