Author: Dr. Marc Glickman
Institution: EnVVeno Medical, Chief Medical Officer and Senior Vice President
Summary
This presentation reviews the development and application of the VenoValve and enVVe® systems by EnVVeno Medical for patients with chronic venous insufficiency (CVI). The VenoValve, an FDA-approved innovative single-valve bioprosthesis, is designed to improve venous reflux in CVI patients. The company is also advancing the enVVe® system, a catheter-delivered venous valve replacement device aimed at refractory CVI cases. Studies show that VenoValve has demonstrated high safety and efficacy in clinical trials, significantly enhancing the quality of life for CVI patients.
Clinical Data on VenoValve
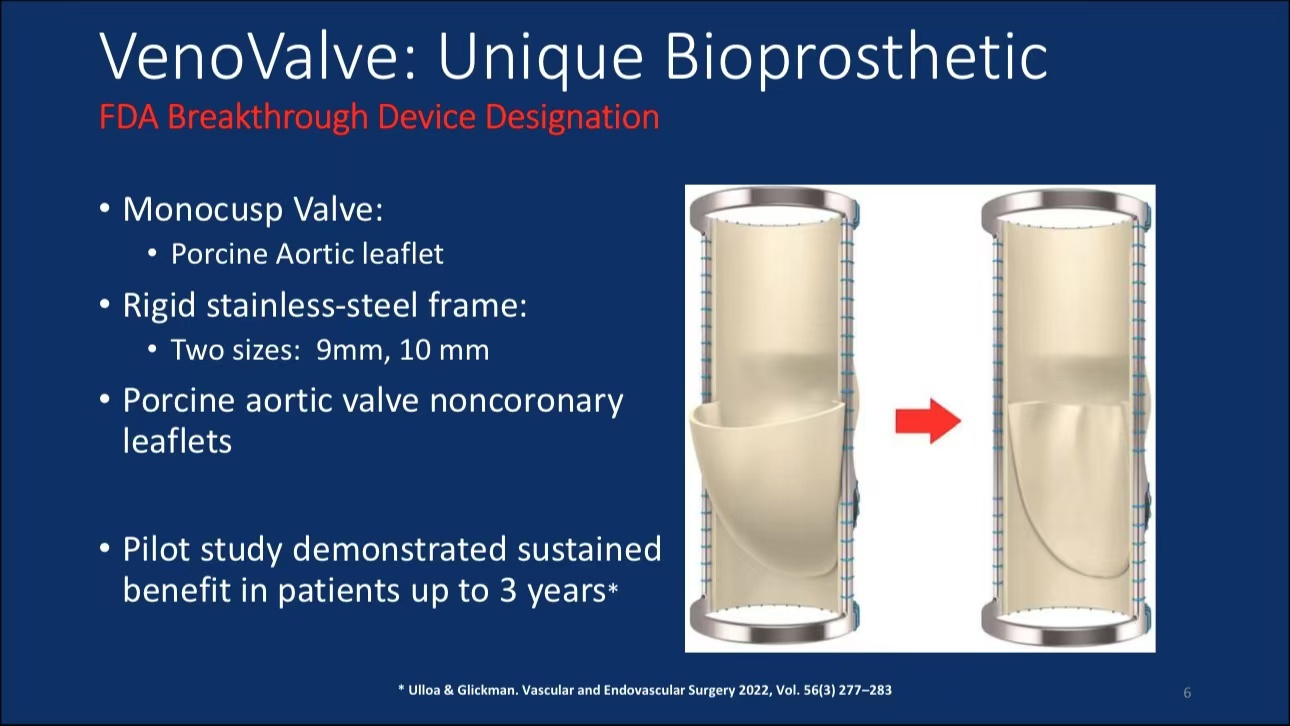
•Device Features: VenoValve uses a rigid stainless steel frame and porcine aortic valve structure, available in 9 mm and 10 mm sizes to meet various anatomical needs.
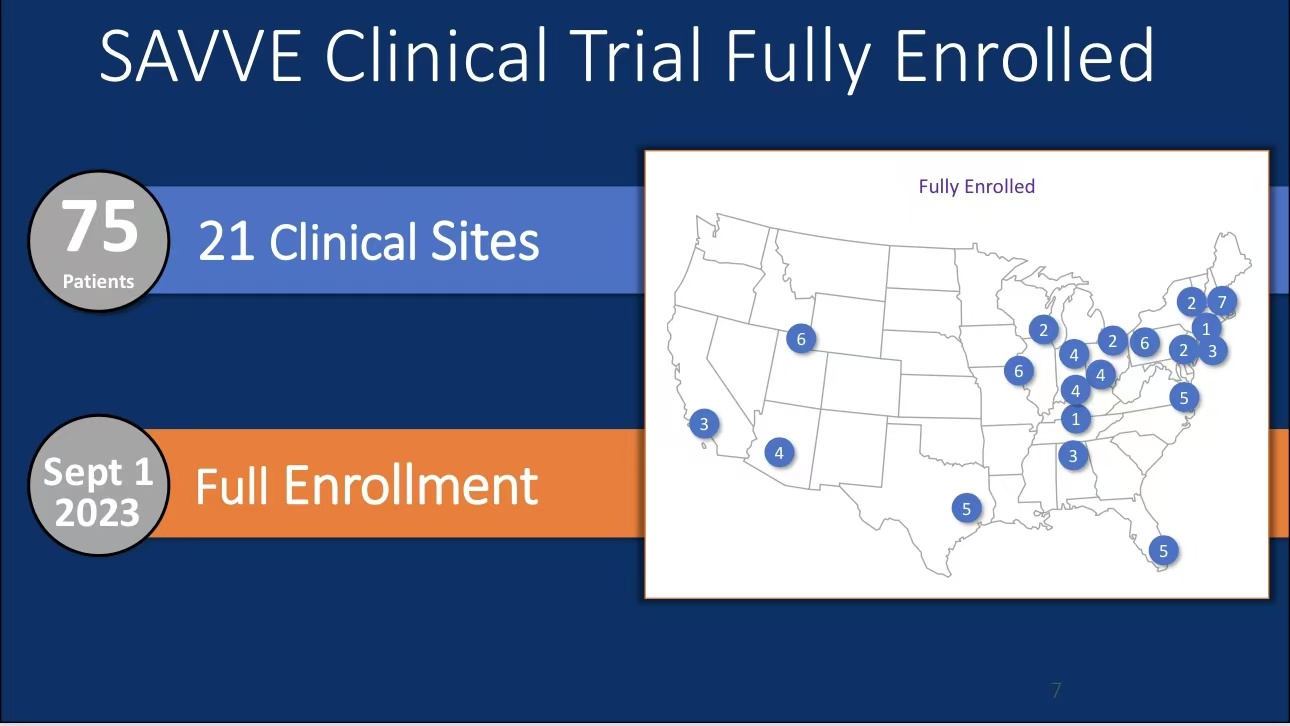
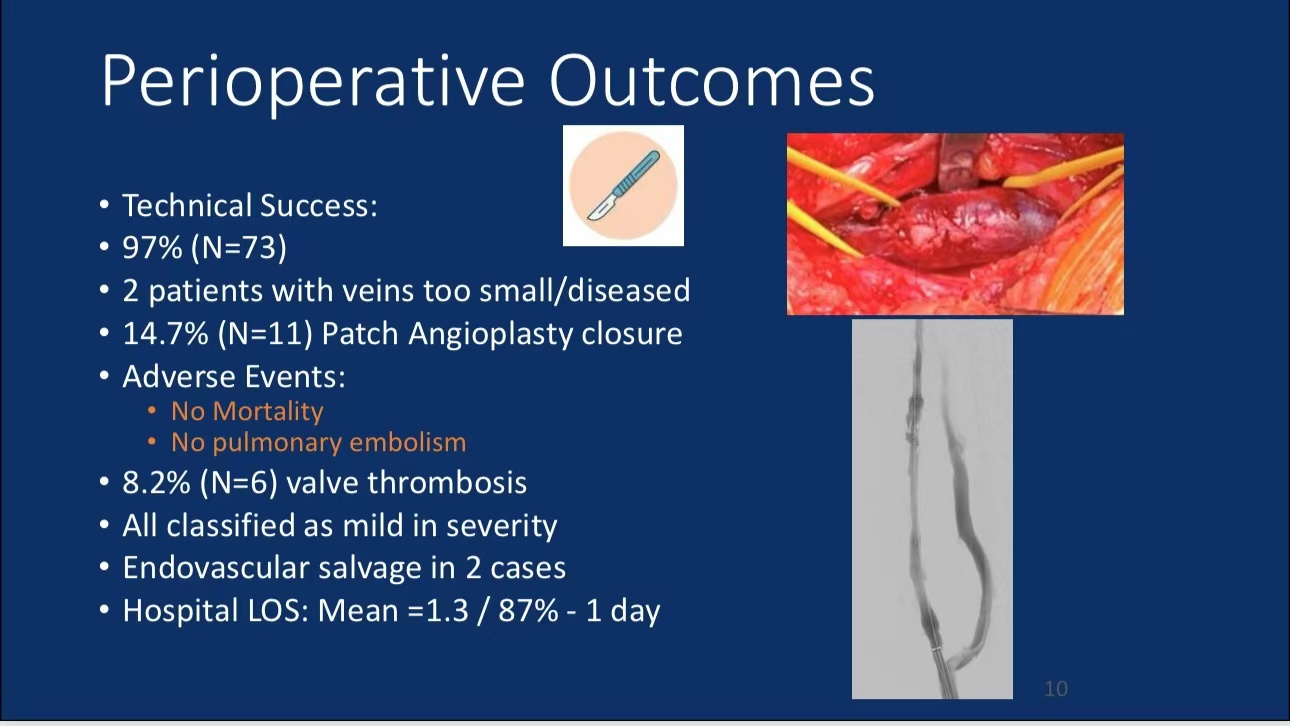
•SAVVE Clinical Trial: The trial enrolled 75 patients, with complete enrollment and data collection finalized in September 2023. Results showed a 97% technical success rate with no deaths or pulmonary embolisms, and significant ulcer healing was observed.
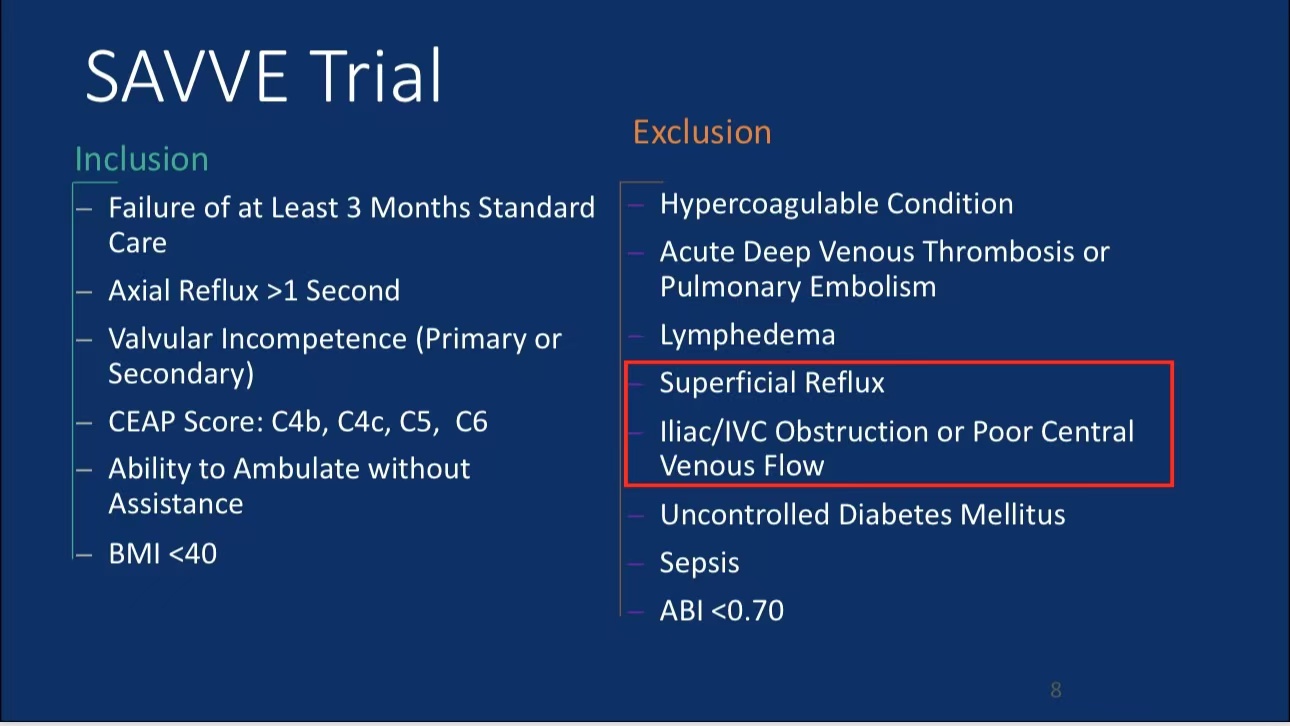
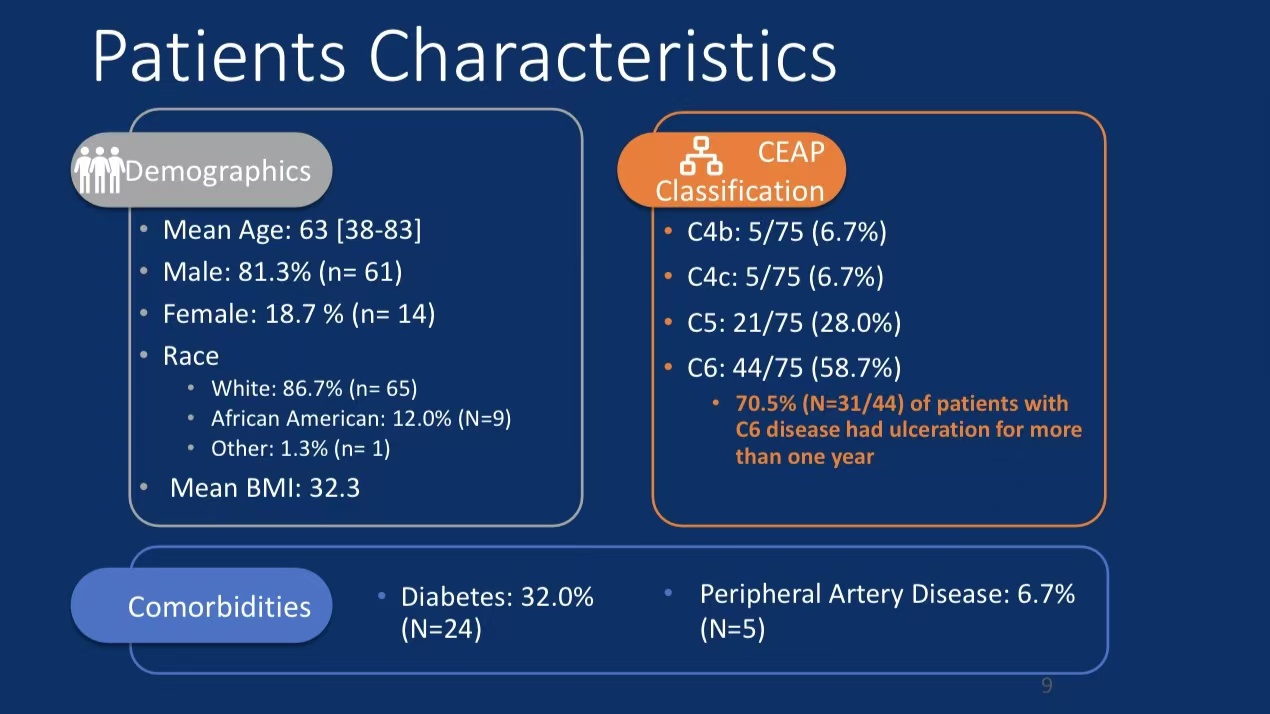
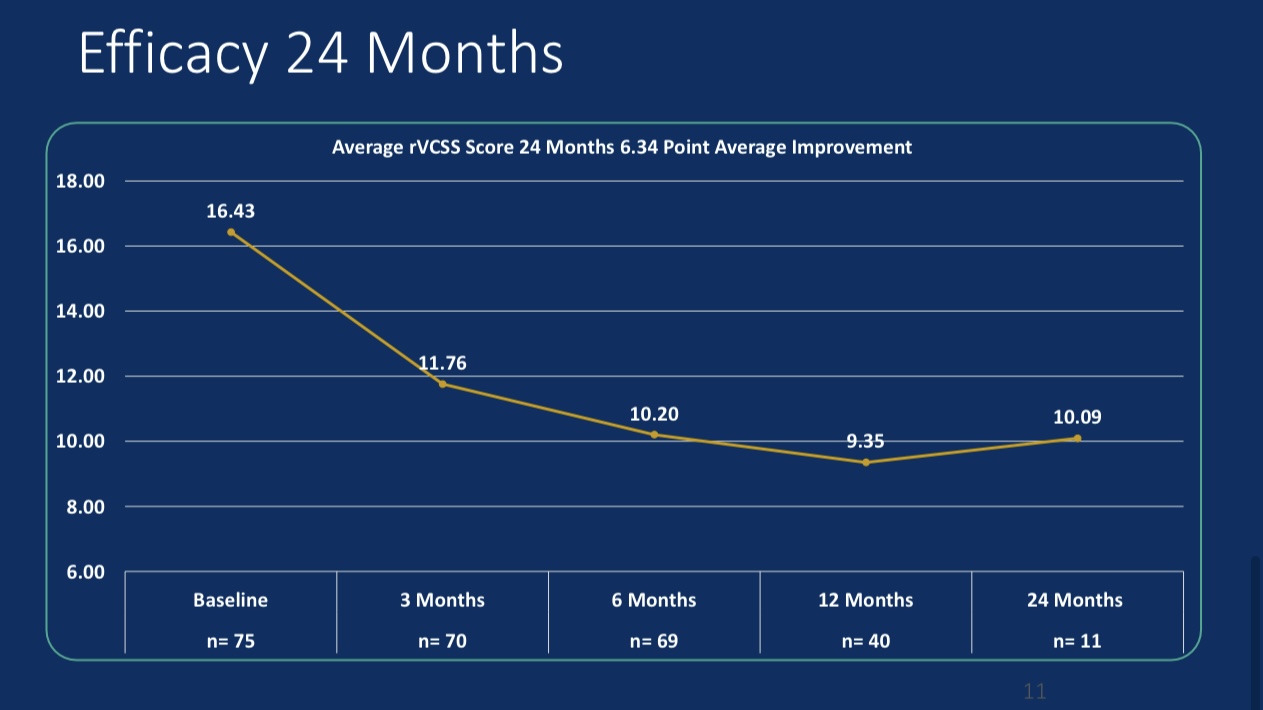
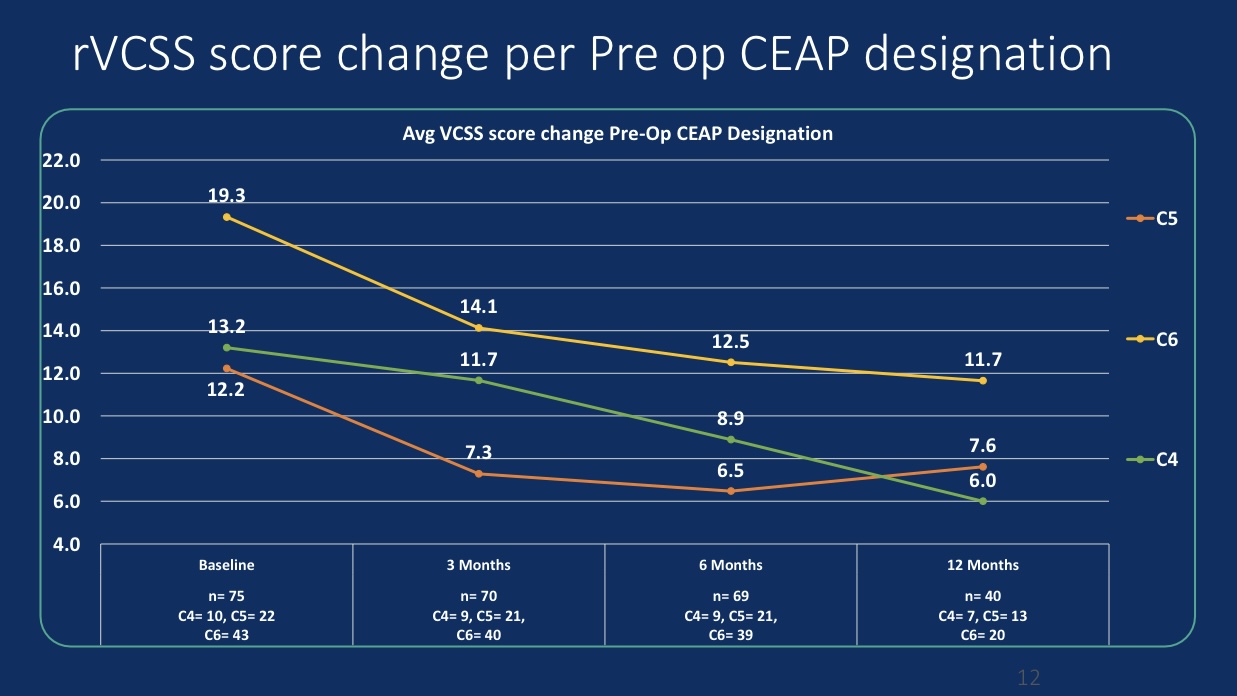
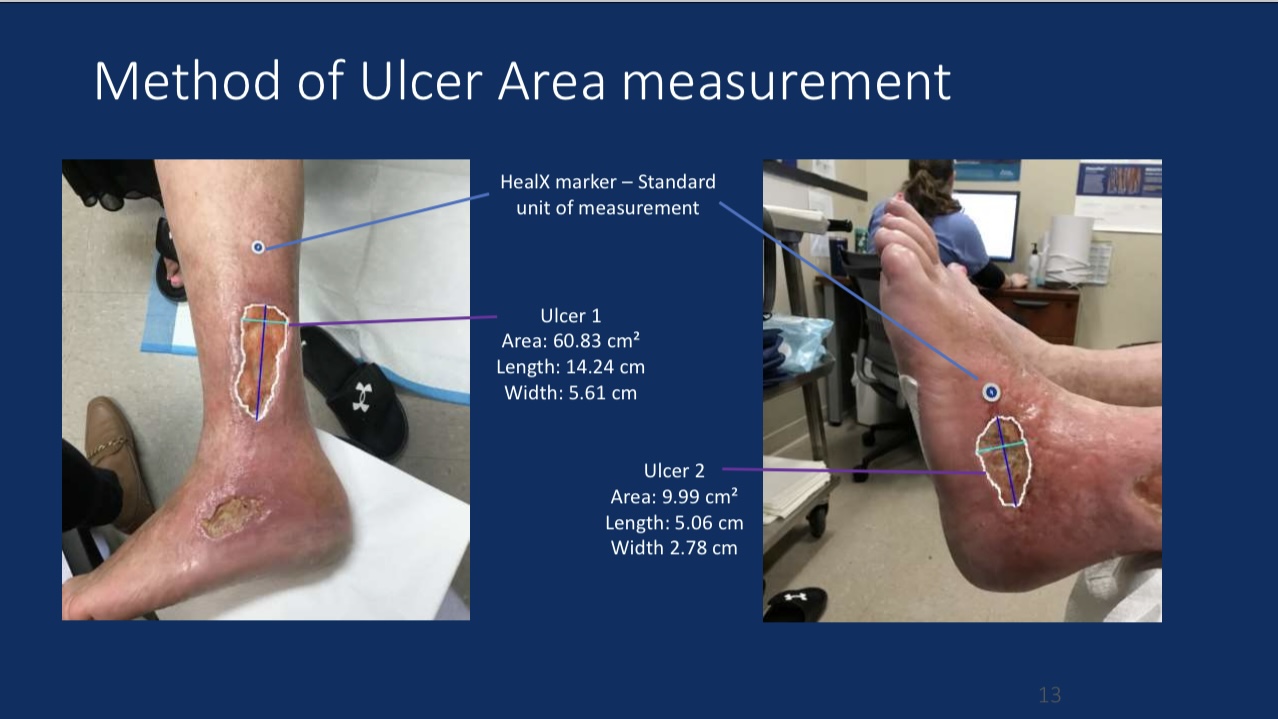
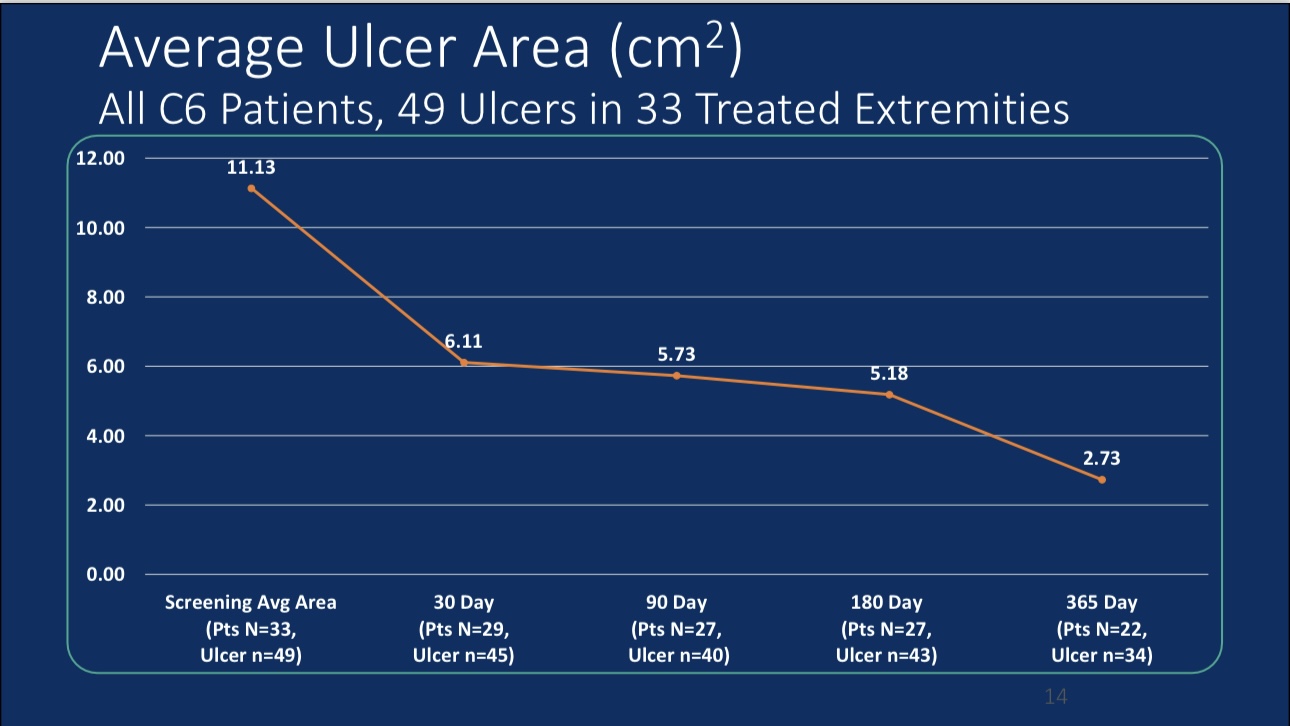
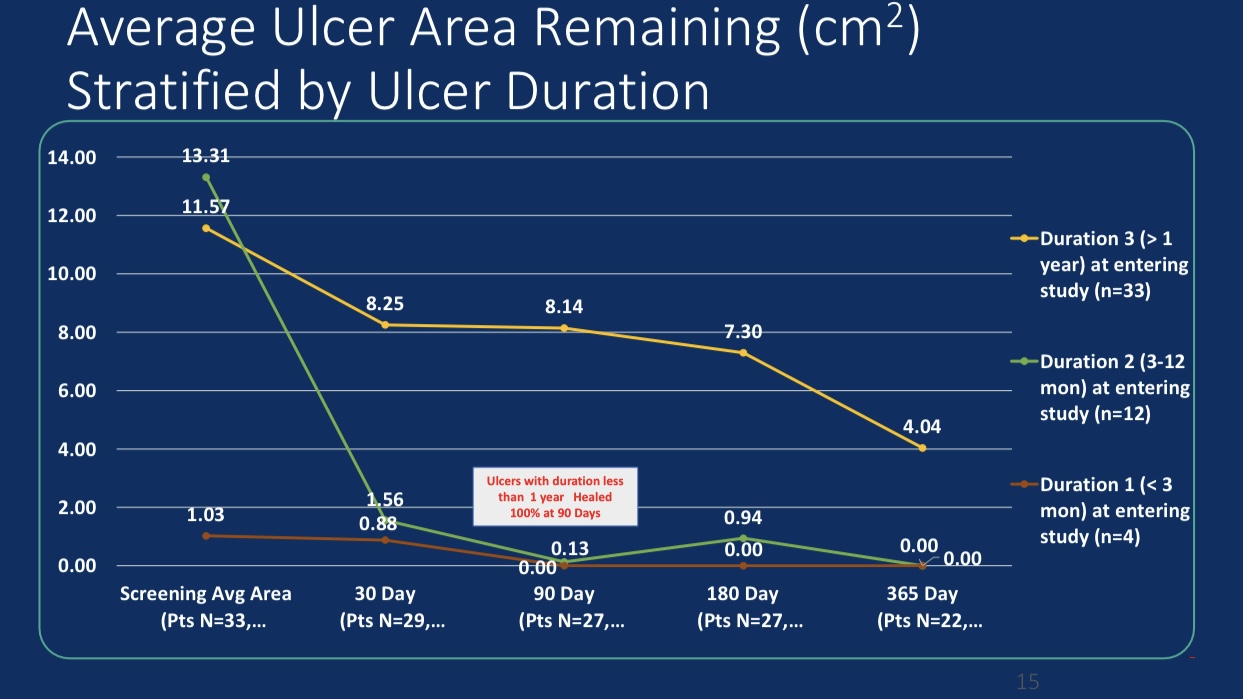
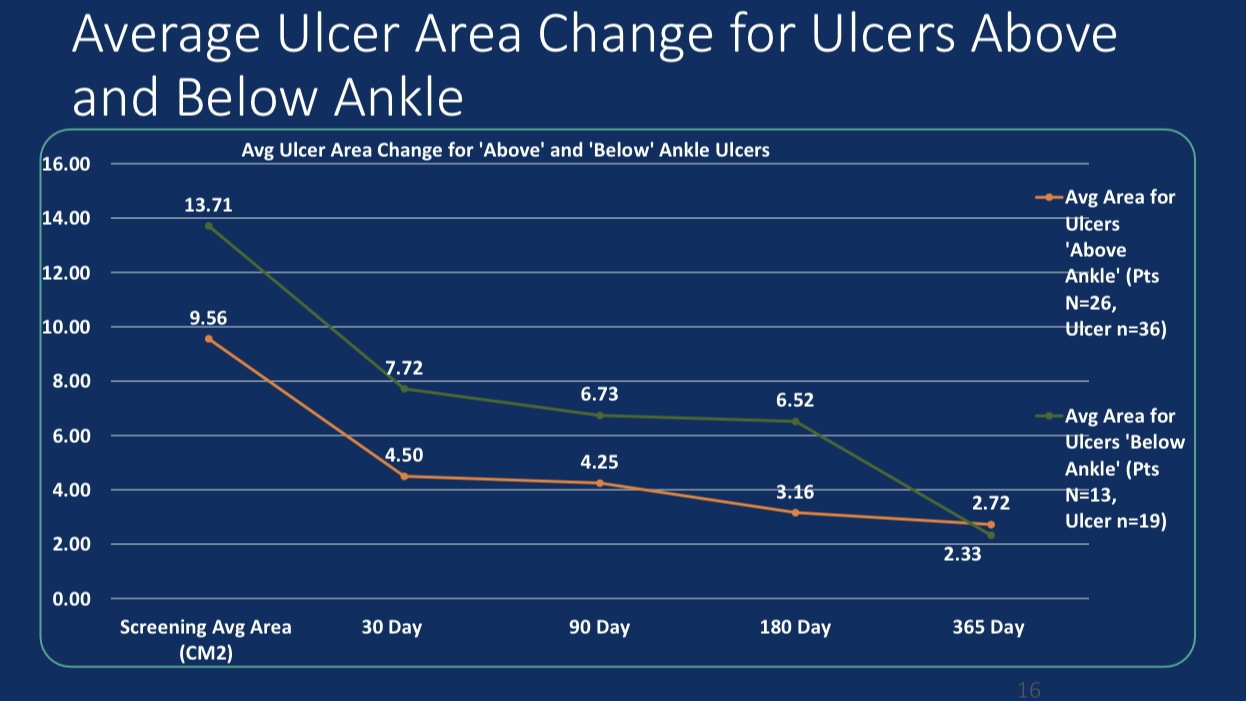
•Efficacy Evaluation: At 24-month follow-up, patients’ revised Venous Clinical Severity Score (rVCSS) decreased by an average of 6.34 points, and C6 patients saw ulcer area reduction to 2.73 cm² within one year, indicating substantial ulcer healing.

Progress on enVVe® System
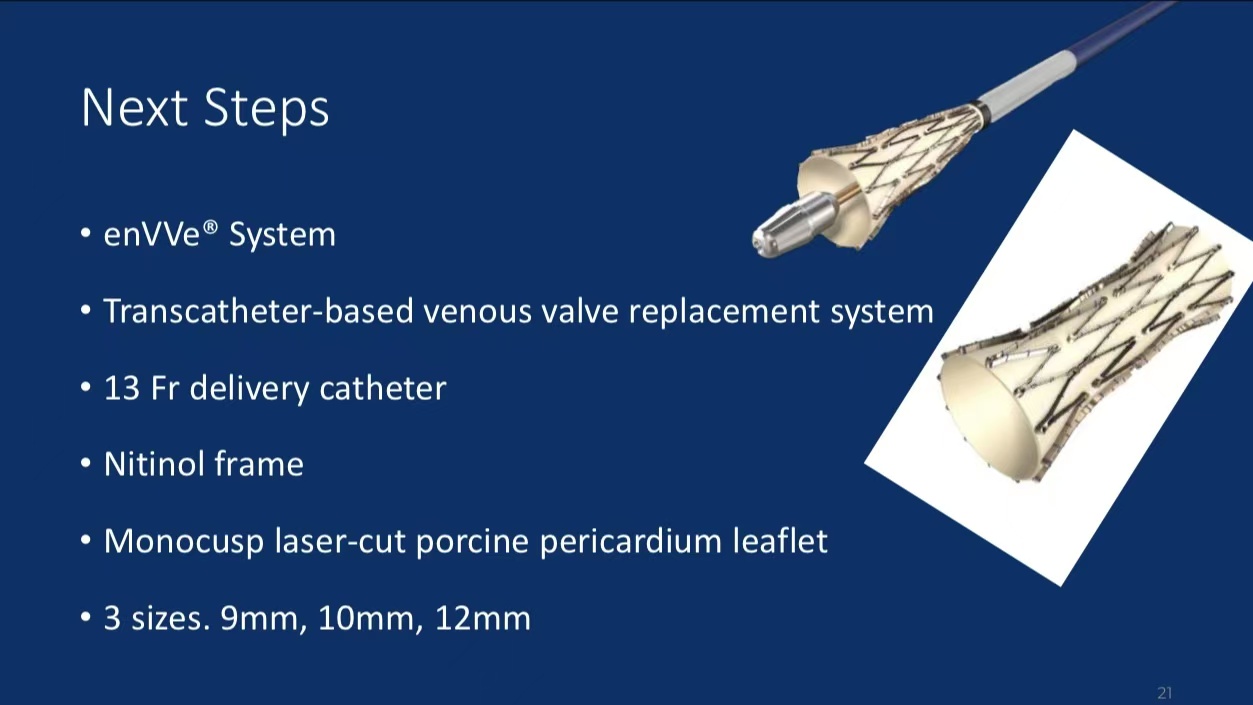
• Device Design: The enVVe® system is a 13 Fr catheter-delivered venous valve replacement device with a nitinol frame and a laser-cut porcine pericardial leaflet, designed for long-term venous reflux support in CVI patients.
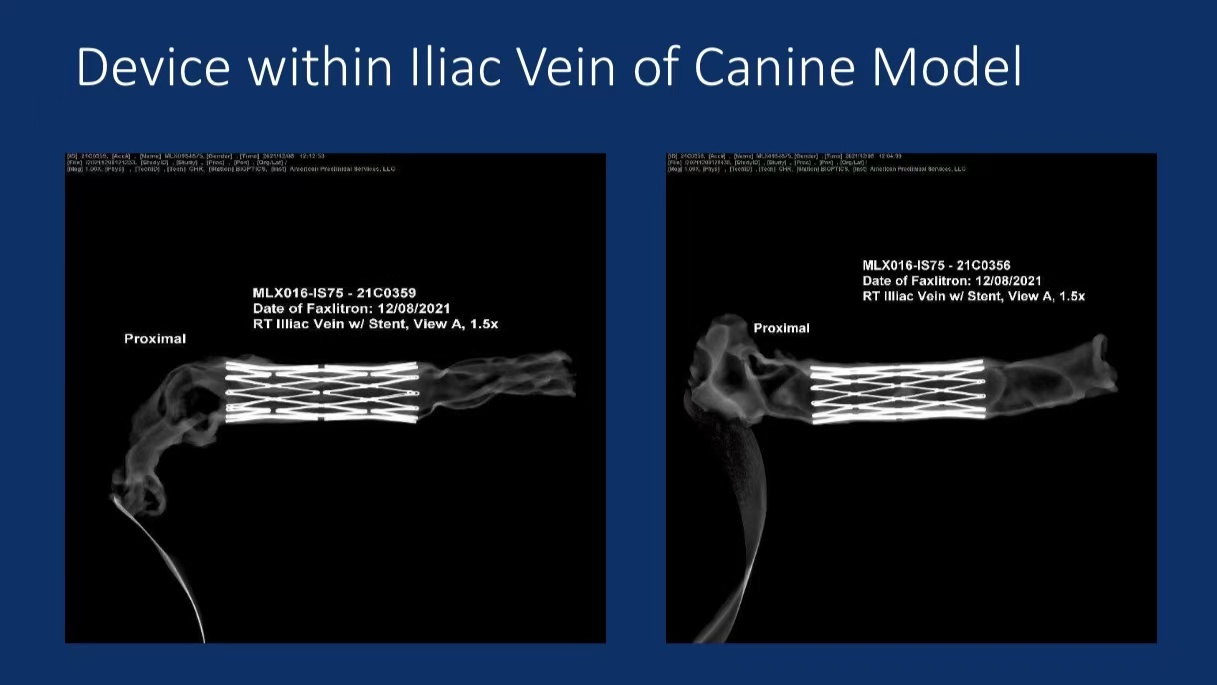
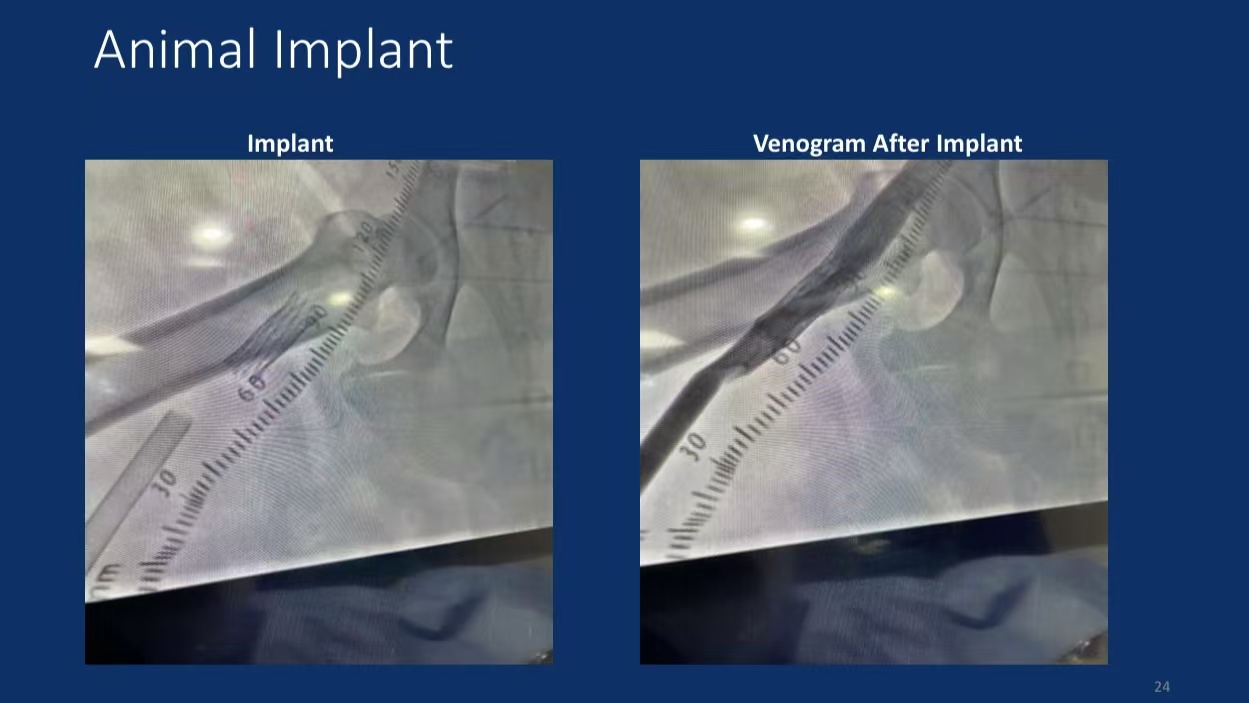
• Animal Study Data: In canine models, the enVVe® system showed good tolerance, with 3-month follow-up results indicating stable maintenance of venous reflux function.
Next Steps
EnVVeno plans to submit the VenoValve’s PMA application by September 2024 and aims to complete the IDE application for the enVVe® device by early 2025 to initiate pivotal trials. The company also plans to raise $20 million to $40 million to support device trials and commercialization.


Conclusion
1.The VenoValve and enVVe® systems offer new treatment options for CVI patients, especially for severe venous reflux.
2.VenoValve has demonstrated significant safety and efficacy in clinical trials, with the upcoming PMA approval expected to further its clinical use.
3.The enVVe® system, as a catheter-delivered venous valve replacement, shows potential for use in complex CVI cases, with future animal and pivotal trials planned to gather additional supporting data.
Contact Us
•Email: endovascluar@simtomax.cn
More international information available at:
•Facebook: Vasco Knight
•Instagram: knight_vasco
Let’s safeguard health together and showcase your brilliance to the world!


