Author: Dr. Jan Skowronski
Institution: Fortuna Devices, Inc.
Summary
This presentation introduces the innovative Fortuna Embolic Protection Device (EPD) developed by Fortuna Devices, designed specifically for chronic total occlusion (CTO) procedures in peripheral vasculature. The Fortuna EPD addresses a market gap, catering to large-diameter (5-10 mm) peripheral vessels and integrating both CTO and EPD functionalities into one device. Built on a 0.035 platform and tailored for peripheral vessel anatomy, it differs from traditional coronary and carotid devices.
Market Demand and Technical Advantages
•Unmet Market Need: Currently, there are no EPDs designed for peripheral vessels on a 0.035 platform, leaving existing devices unable to meet anatomical requirements for peripheral vessels, thereby increasing the complexity and embolization risk of CTO procedures.

•Technical Innovation of Fortuna: The device adapts to varying vessel diameters, reducing intraoperative embolization risk. Its design minimizes the likelihood of subintimal entry, making operations more stable and safer for larger peripheral vessels.
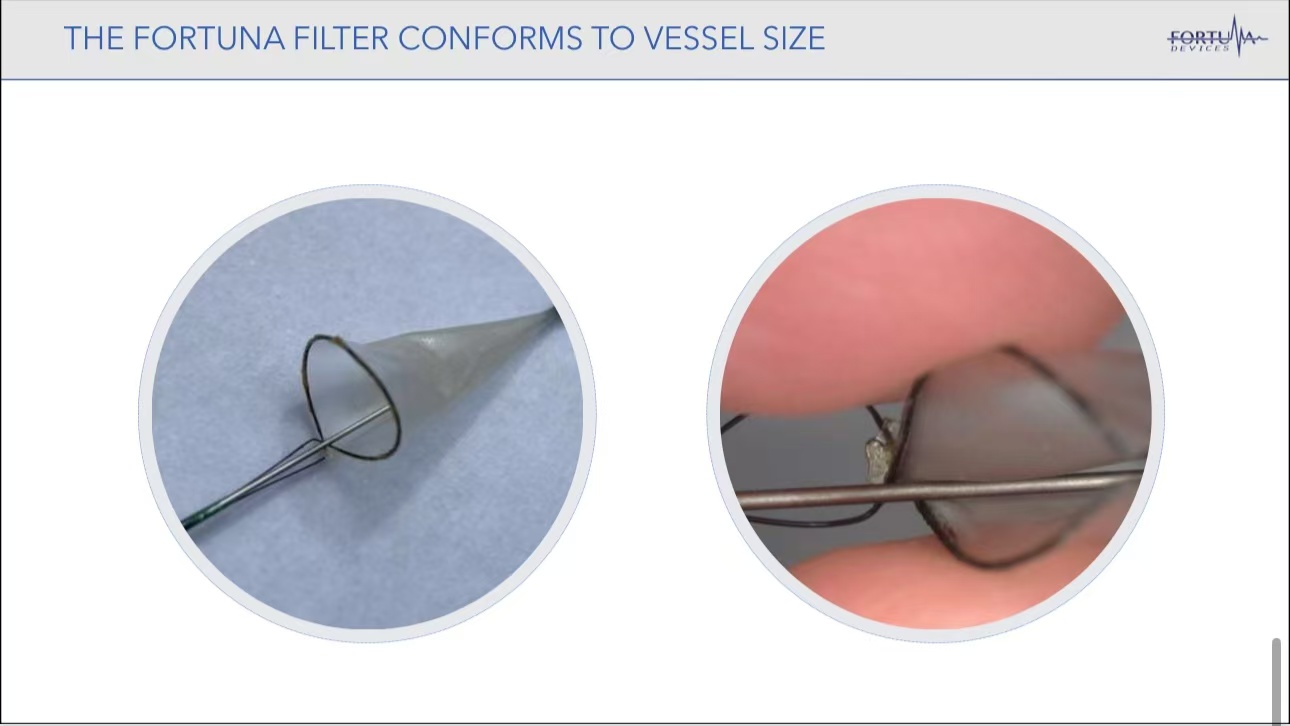
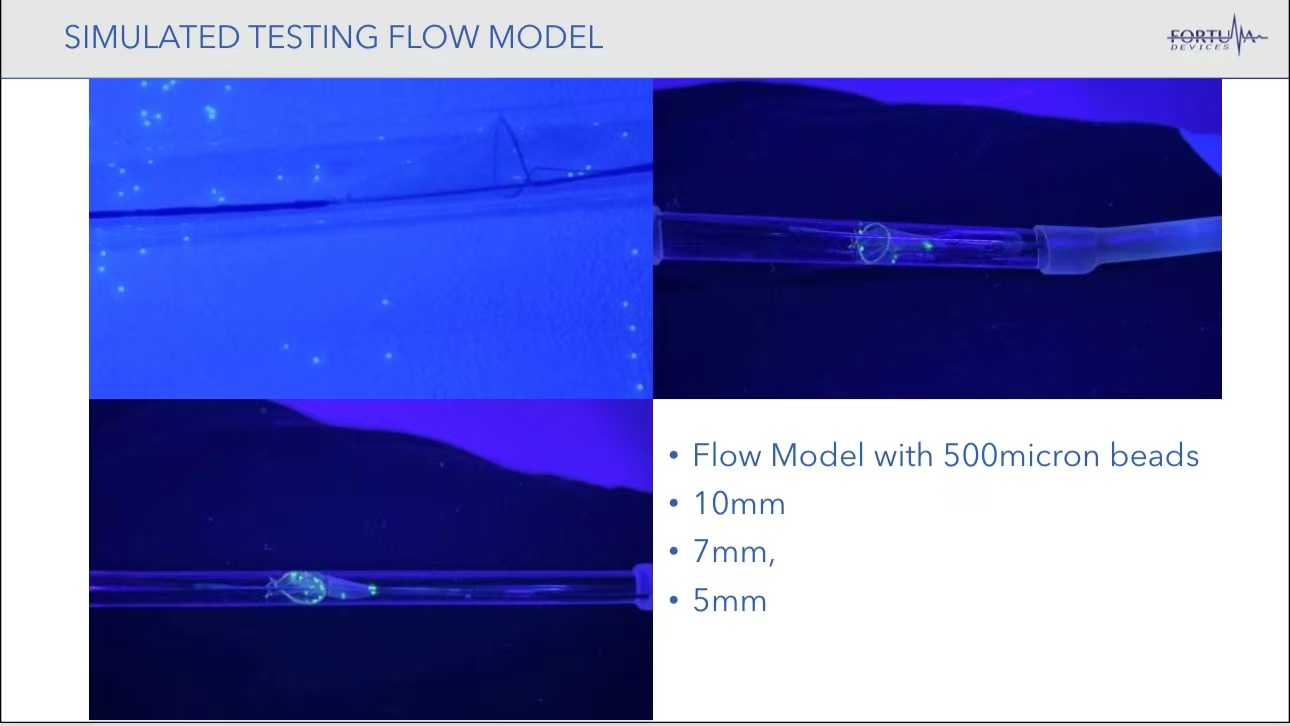
Preclinical Studies and Device Capabilities
•Simulated Testing: In models of 10 mm, 7 mm, and 5 mm diameters, the Fortuna EPD demonstrated excellent blood flow restoration and efficiently captured simulated thrombus particles of 500 microns, showcasing its high embolic protection capability.
•Animal Testing: Experiments conducted at the Texas Heart Institute on pigs confirmed that the device could restore normal blood flow after capturing thrombus, validating its safety and efficacy in complex anatomical environments.

FDA Approval Process and Funding Plan
•FDA Pre-Approval Feedback: In October 2023, the US FDA confirmed Fortuna’s classification under the 510(k) pathway, approving reference to SPIDER FX and Vanguard EPD as predicate devices.

•Clinical Trials and Funding: The company plans to conduct clinical trials with 105-120 patients across the US and other regions, with a 30-day follow-up to assess safety. The ongoing Series A funding round aims to raise $2.5 million to support further development, clinical trials, and the first human trials.

Conclusion
1.The Fortuna EPD, integrating CTO and EPD functionalities, offers an innovative embolic protection solution for peripheral vascular procedures, reducing embolization risks.
2.Preclinical tests and animal studies have demonstrated its safety and efficacy, laying a solid foundation for market entry.
3.Future clinical trial data will further support its application potential in the peripheral vascular field, addressing an existing market need.
Contact Us
•Email: endovascluar@simtomax.cn
More international information available at:
•Facebook: Vasco Knight
•Instagram: knight_vasco
Let’s safeguard health together and showcase your brilliance to the world!


