Presenter: Prof. Thomas Zeller
Affiliation: University Heart Center Bad Krozingen, Germany
Abstract
Prospective multicenter study (n=101) demonstrates: COVERA™ PLUS covered stent achieves 3.0% TLR (p=0.0015 vs 12% performance goal) and 84.8% primary patency at 9 months for iliac stenotic/occlusive disease.
Introduction
Iliac artery disease carries high restenosis risk, with bare-metal stents showing >30% reintervention at 5 years. BD's COVERA™ PLUS stent combines nitinol skeleton with ePTFE membrane to inhibit neointimal hyperplasia, offering a novel solution for complex lesions.
Key Findings (Prospective Multicenter Trial)
1.Patient Profile
101 patients (mean age 61.3), 64.4% smokers, 79.2% hypertension
Lesions: 77.5% stenosis (mean 85.8% occlusion), 22.5% total occlusion
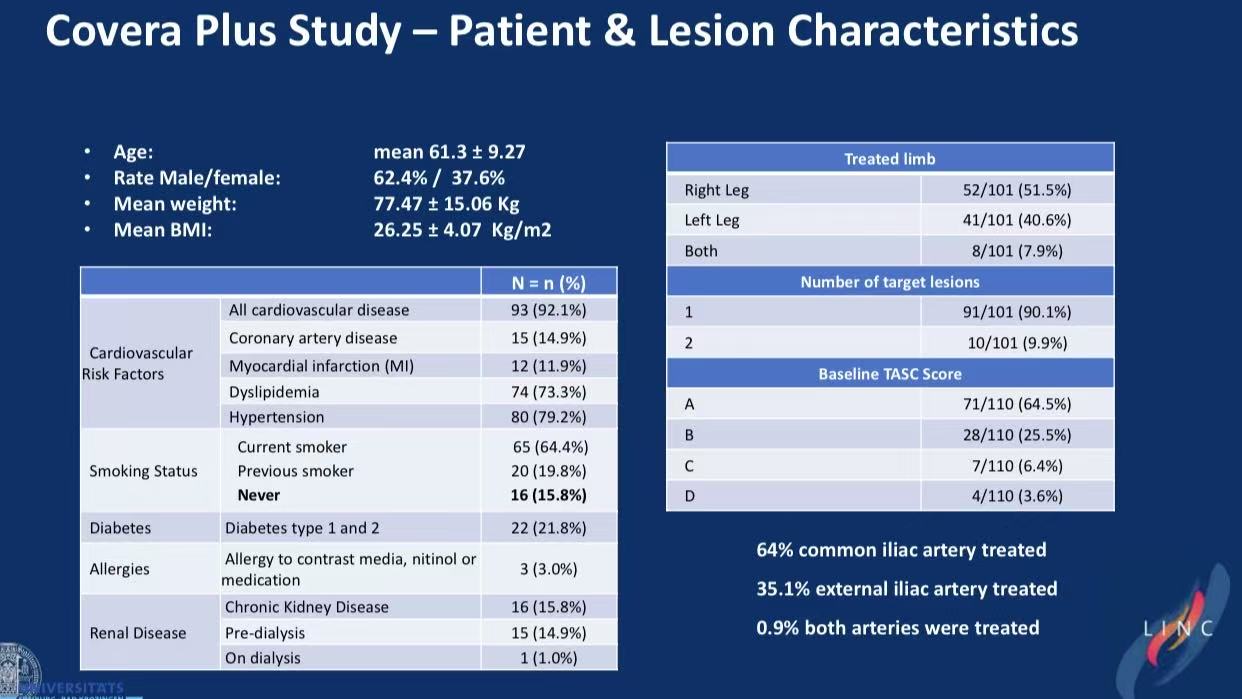
TASC Class: 90% A/B (64.5% A + 25.5% B)
64% common iliac, 35.1% external iliac involvement
2.Procedural Excellence
97.2% pre-dilation, 99.1% post-dilation
Mean stent diameter 8.4mm, length 50.8mm
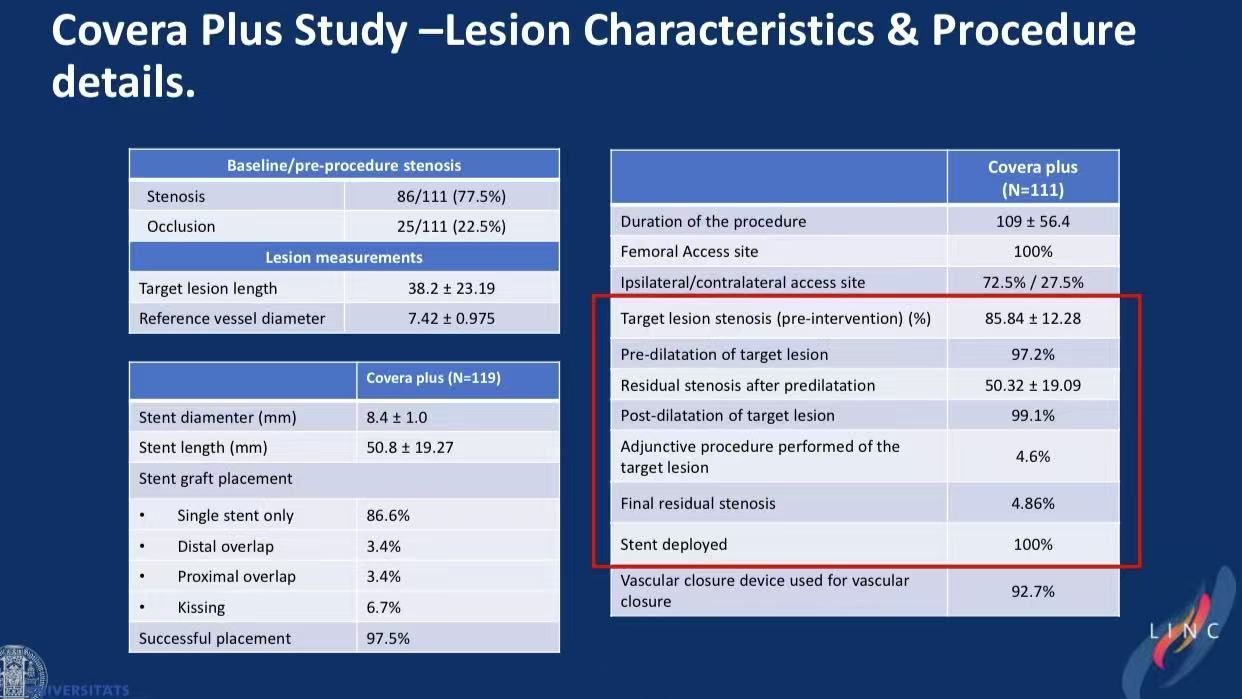
Kissing stents in 6.7%
97.5% technical success, 4.86% residual stenosis
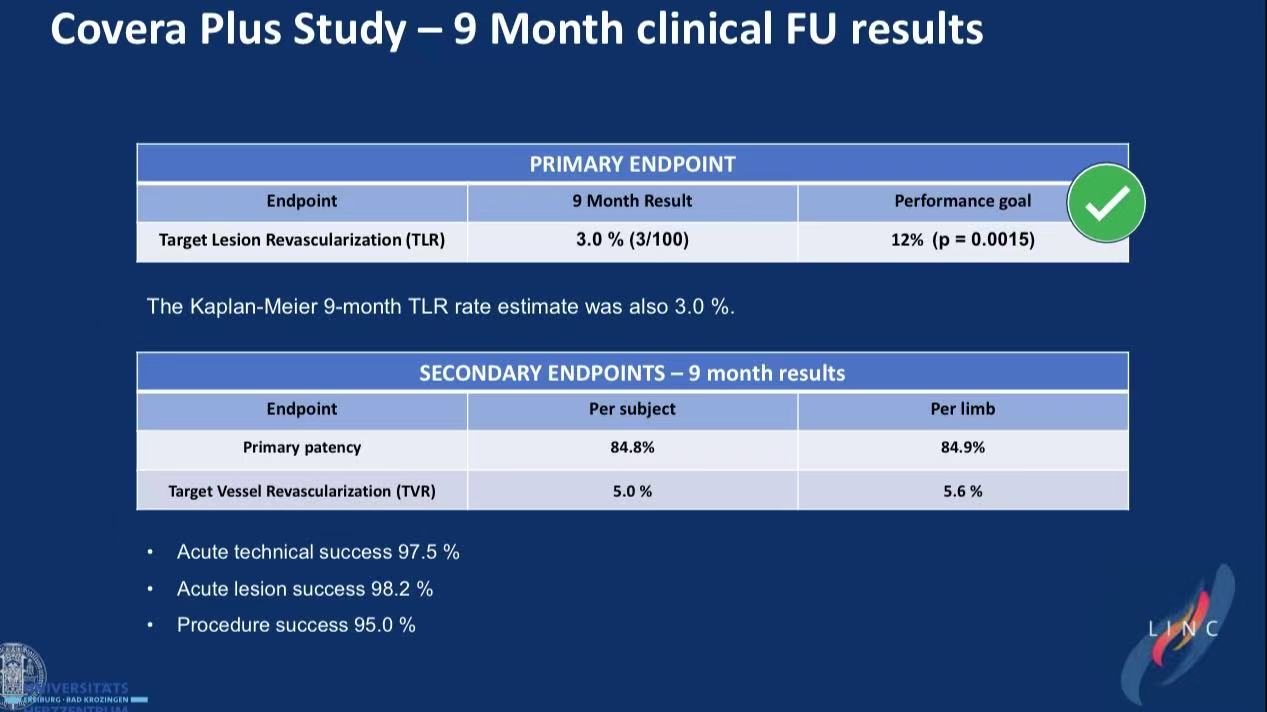
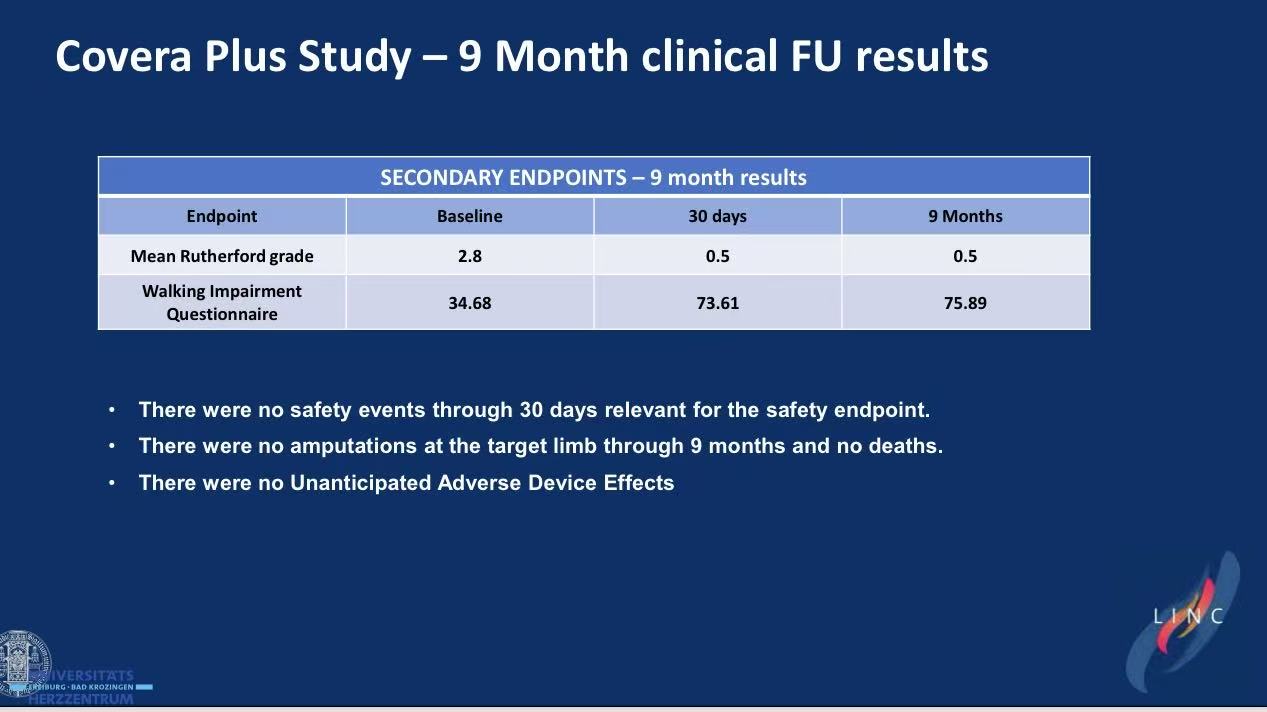
3.9-Month Efficacy
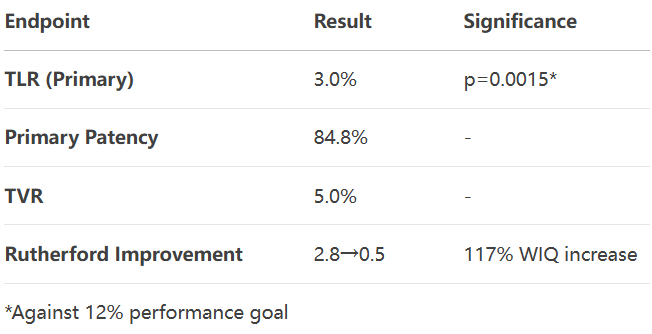
4.Safety Milestones
Zero device-related death/MI/amputation at 30 days
No unanticipated device effects through 9 months
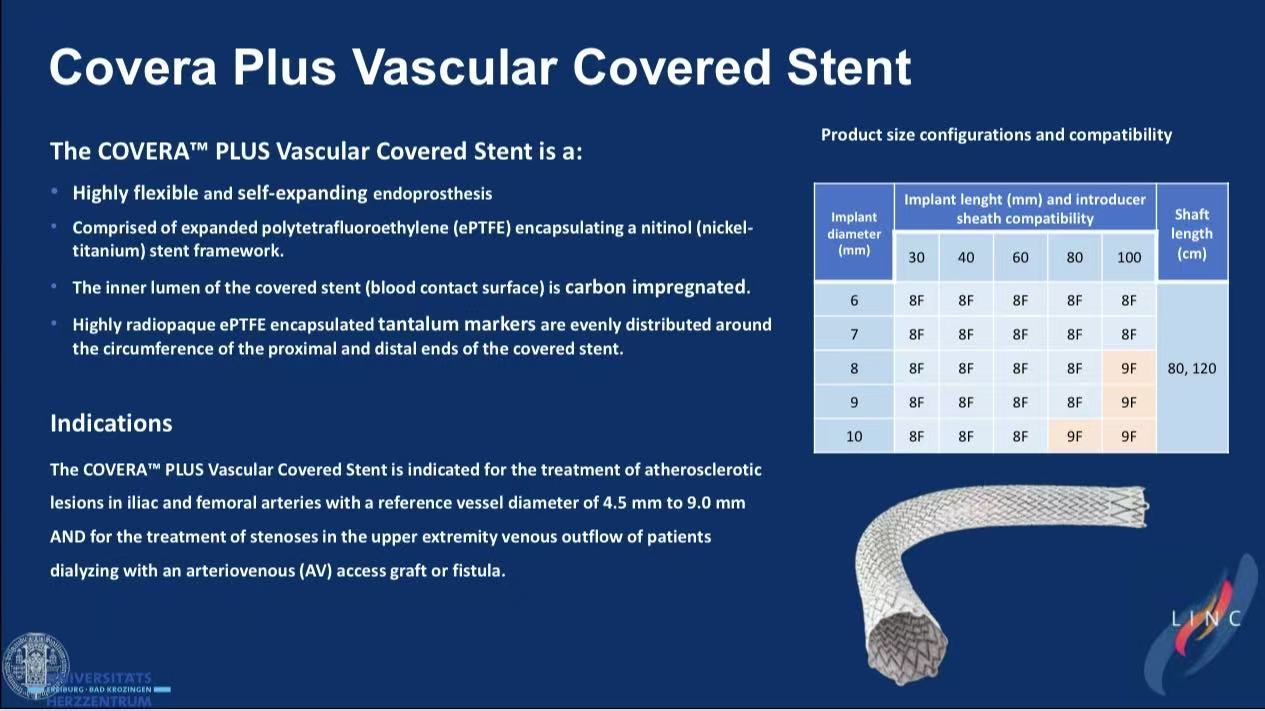
COVERA™ PLUS Advantages
1.Structural Innovation
Dual-layer: Nitinol framework + ePTFE membrane (suppresses hyperplasia)
Carbon coating: Blood-contact surface reduces thrombogenicity
Precise deployment: Tantalum markers at both ends enhance visibility
2.Clinical Versatility
Diameter range: 4.5-10mm
Configurations: Straight (outflow≤inflow) / Flared (outflow>inflow) designs
Delivery: 8-9Fr sheath compatibility minimizes access injury
Conclusions
1.Short-term superiority: 3% TLR sets new benchmark, ideal for TASC A/B lesions
2.Long-term evidence pending: 24/36-month data will validate durability in occlusions
3.Technical imperative: Near-universal post-dilation ensures optimal apposition
Clinical Note: TASC C/D lesions represented <10%, warranting larger cohort validation.



