Presenter: Lieven Maene, MD
Hospital: OLV Hospital Aalst, Belgium
Abstract
This article examines the clinical value of bare-metal stents (BMS) in complex aortoiliac occlusive disease (AIOD) through evidence-based medicine, integrating long-term data from covered stents (Advanta V12) to guide therapeutic decision-making.
Introduction
The treatment paradigm for iliac artery disease is evolving. With advancements in drug-coated balloons (DCB), covered stents (CS), and vessel preparation techniques (e.g., IVL), the role of bare-metal stents (BMS) requires redefinition. Prof. Lieven Maene (OLV Hospital, Belgium) presents critical evidence supporting the irreplaceable value of BMS in contemporary practice.
Core Research Findings
Technological Evolution (2017-2023)
2017: BMS-dominated era
2018: DCB+BMS combination therapy
2019: Emergence of covered stents (CS)
2023: BMS resurgence in selected scenarios (e.g., calcified focal lesions)
Key Evidence: BMS vs CS
ESC 2023 Meta-Analysis (1,182 patients):
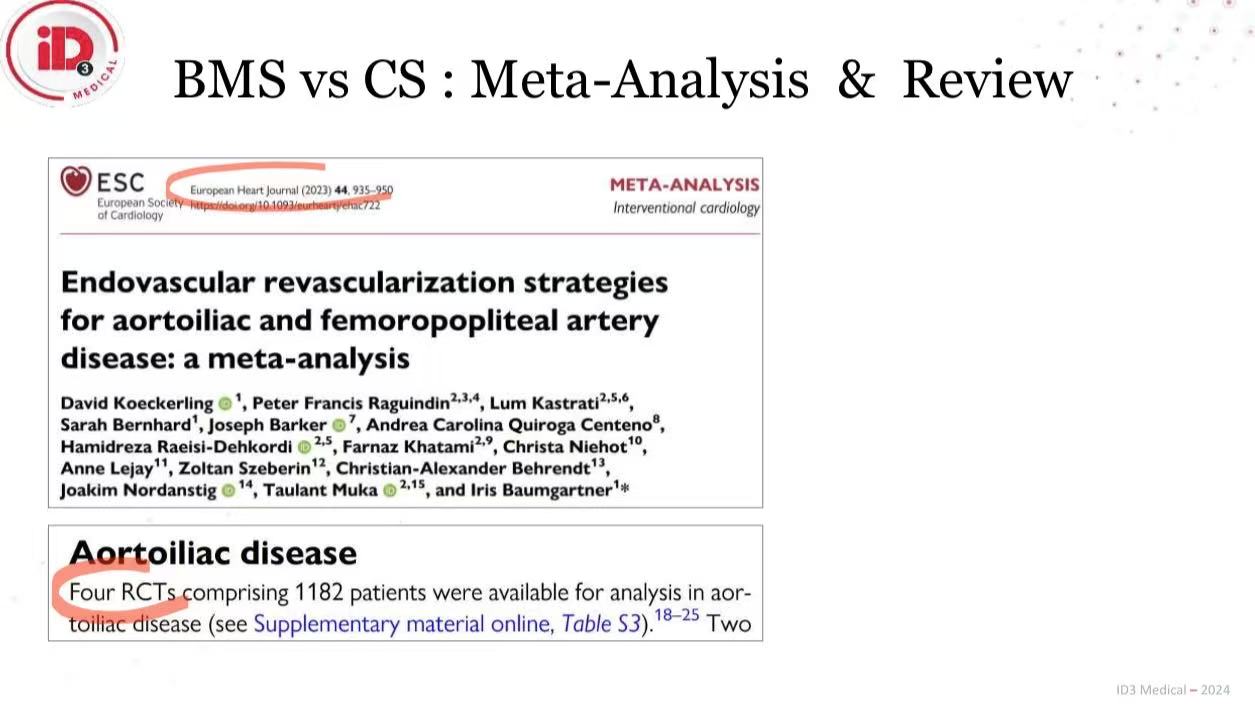
No single-device strategy demonstrated superiority in aortoiliac disease

CS and BMS show scenario-dependent differences in patency/safety
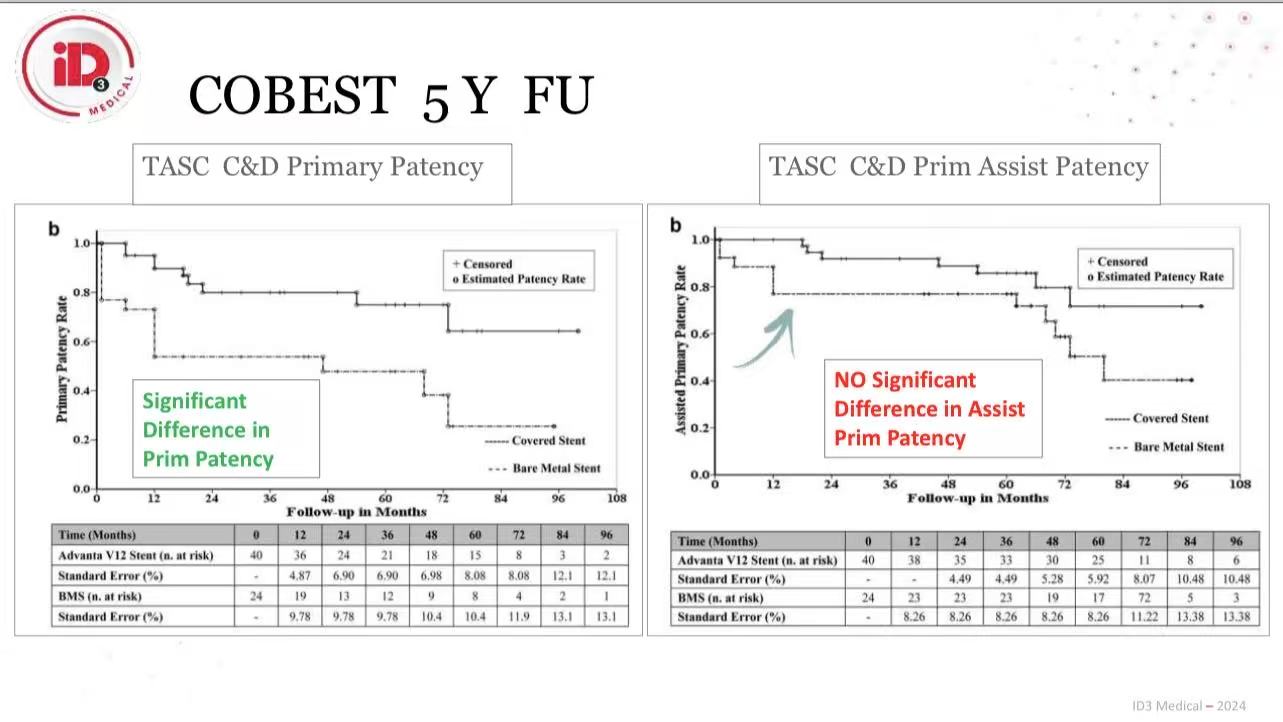
Unique Advantages of BMS
Collateral Preservation: Open-cell design maintains flow to hypogastric arteries
Mechanical Adaptability: Self-expanding BMS resists fracture in tortuous external iliac arteries (EIA)
Bailout Utility: Combined with CS for rupture management

Product Highlights: Advanta V12 Covered Stent
1.Material Innovation: ePTFE membrane reduces neointimal hyperplasia vs BMS
2.Precise Deployment: Radiopaque markers enhance accuracy in bifurcation lesions
3.Long-term Evidence: Only balloon-expandable CS with 5-yr RCT data
4.Regulatory Endorsement: 2024 EU MDR certification (highest EU medical device standard)
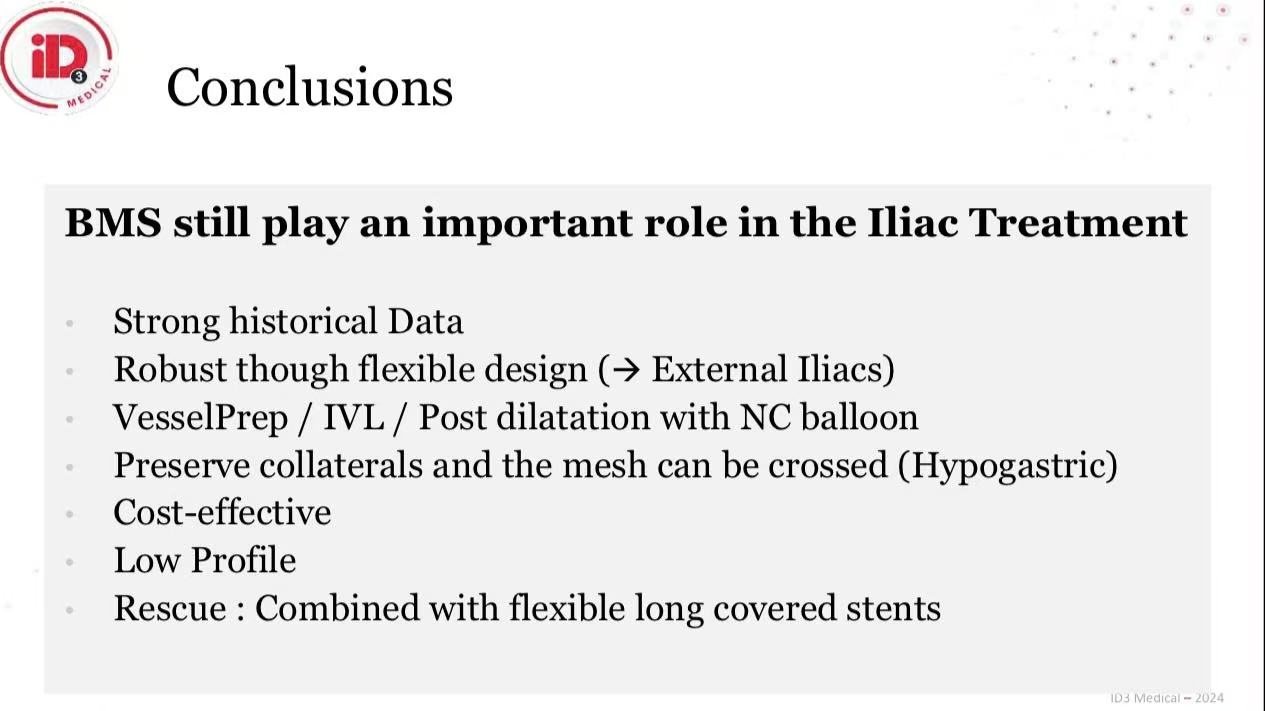
Conclusions
1.Stratified Therapeutic Approach:
TASC C/D lesions: Advanta V12 as first-line
Calcified focal lesions: IVL + BMS to avoid over-stenting
Tortuous EIA segments: Flexible self-expanding BMS preferred
2.Health Economic Value: BMS remains cost-effective for non-complex disease
3.Future Directions: Stent-free strategies (VesselPrep + DCB) under clinical investigation