Abstract
Addressing long-term safety concerns of paclitaxel devices and biomechanical challenges in peripheral arteries, the novel bioresorbable Efemoral™ scaffold achieves durable patency for diffuse femoropopliteal lesions through segmented design and directional sirolimus release, earning FDA Breakthrough Device designation.
Introduction: Paclitaxel Safety Controversy and Sirolimus Advancement
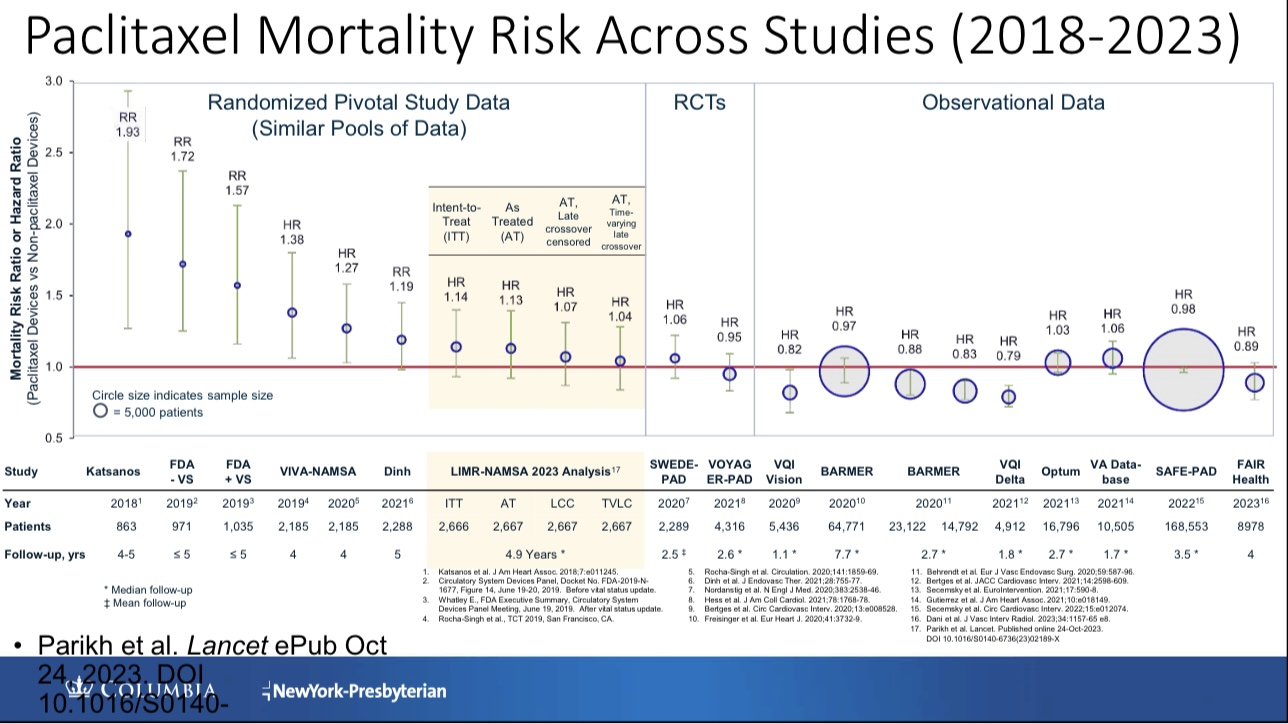
The 2018 Katsanos study suggested a 68% increase in 2-year mortality risk with paclitaxel devices (RR 1.68), rising to 93% at 5 years (RR 1.93). Subsequent analyses found no mortality signal, but sirolimus emerged as a safer alternative due to lower toxicity . However, peripheral arteries demand innovative solutions for bending mechanics and drug distribution —prompting the development of the Efemoral™ Vascular Scaffold System.
Research Insights: Efemoral™ Solving Three Critical Challenges
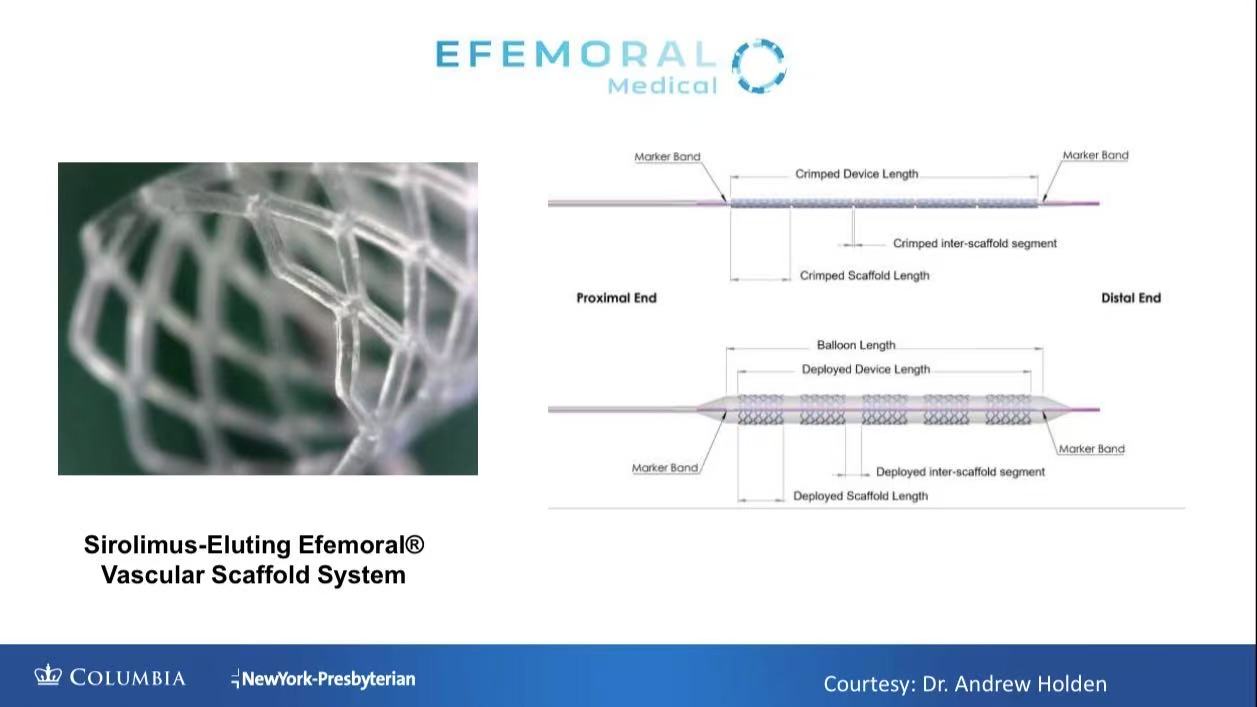
1. Biomechanical Adaptation
FlexStep Technology: Multiple short scaffolds with inter-segment spacing accommodate vessel bending (Animal data: 113° femoral flexion without collapse).
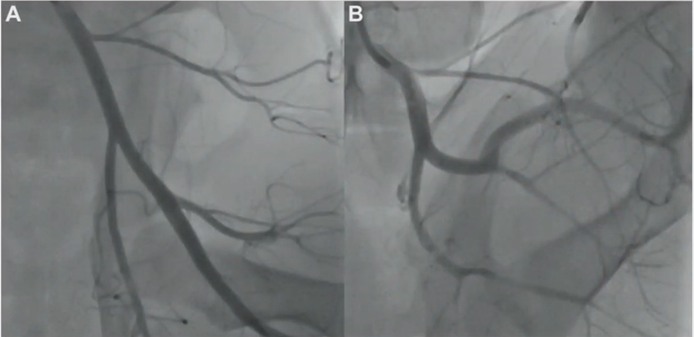
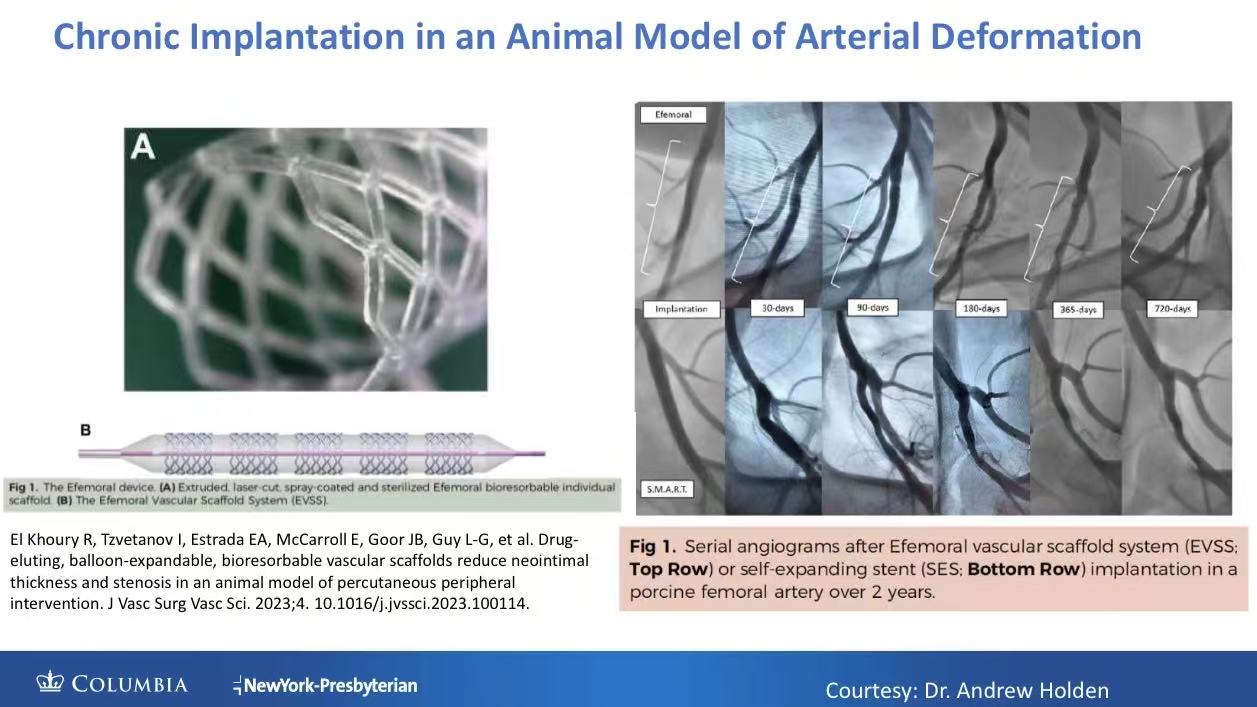
Bioresorbable PLA Framework: Complete absorption in 2 years, eliminating metal fatigue risk.
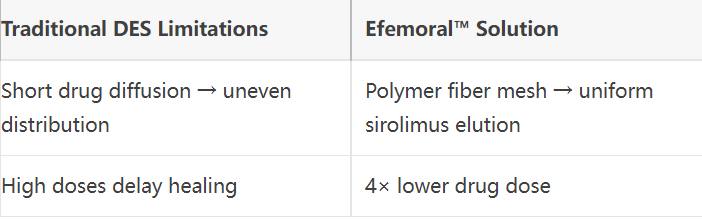
2. Drug Delivery Optimization
3. Clinical Validation
MAGICAL Trial Design:
BTK Cohort: 368 CLTI patients
SFA Cohort: 478 patients (Direct comparison with paclitaxel DCB)
Nano-Enhanced Efficacy: Dual-drug nanoparticles (SirPlux Duo) reduce sirolimus IC₅₀ by 3-fold.
Key Innovations: FDA Breakthrough Device Highlights
Dynamic Support: Inter-scaffold segments distribute bending stress (Reference: 15% arterial shortening vs 20% native).
Precise Drug Delivery: Sirolimus elution via PLA mesh sustained over 90 days .
Complete Absorption: No permanent implant after 2 years.
Broad Applicability: Suitable for long lesions (>10cm), calcified, and joint-crossing segments (Reference: 97mm porcine implantation).
Technical Note: Efemoral™'s elastic modulus matches native vessels, validated by micro-CT (Reference article).
Conclusion: Clinical Pathway Forward
1.Immediate Practice: Prioritize sirolimus devices for CLTI/long lesions (Efemoral™ > paclitaxel DCB).
2.Future Horizon: Bioresorbable scaffolds with nanotechnology will dominate next-decade interventions.
3.Pivotal Evidence: MAGICAL trial 5-year outcomes will confirm sirolimus durability.
4.Clinical Insight: For femoropopliteal lesions crossing joints, select devices combining biomechanical adaptability and sustained drug release to reduce reintervention.



