Presenter: Angelo Cioppa MD
Institution: Montevergine Clinic, Mercogliano, Italy
Abstract
Addressing safety concerns of paclitaxel devices (aneurysmal degeneration/HALO), the polymer-free sirolimus-eluting self-expanding DES (NITIDES™) demonstrates efficacy in 77 high-complexity femoropopliteal cases, offering a novel solution for calcified, long-segment, and CLI lesions.
Introduction: Safety Challenges of Paclitaxel Devices
Recent studies indicate potential risks of paclitaxel-coated devices (DCB/DES), including aneurysmal degeneration (DCB) and HALO phenomena (DES) (Katsanos et al., 2018; Zenunai et al., 2023). For long lesions (>15 cm), severe calcification, and chronic limb-threatening ischemia (CLTI), device safety and durability remain challenging. NITIDES™ DES, featuring a polymer-free platform + sirolimus-fatty acid composite (Amphilimus™), emerges as a promising alternative.
Study: Real-World Data of Complex Lesions

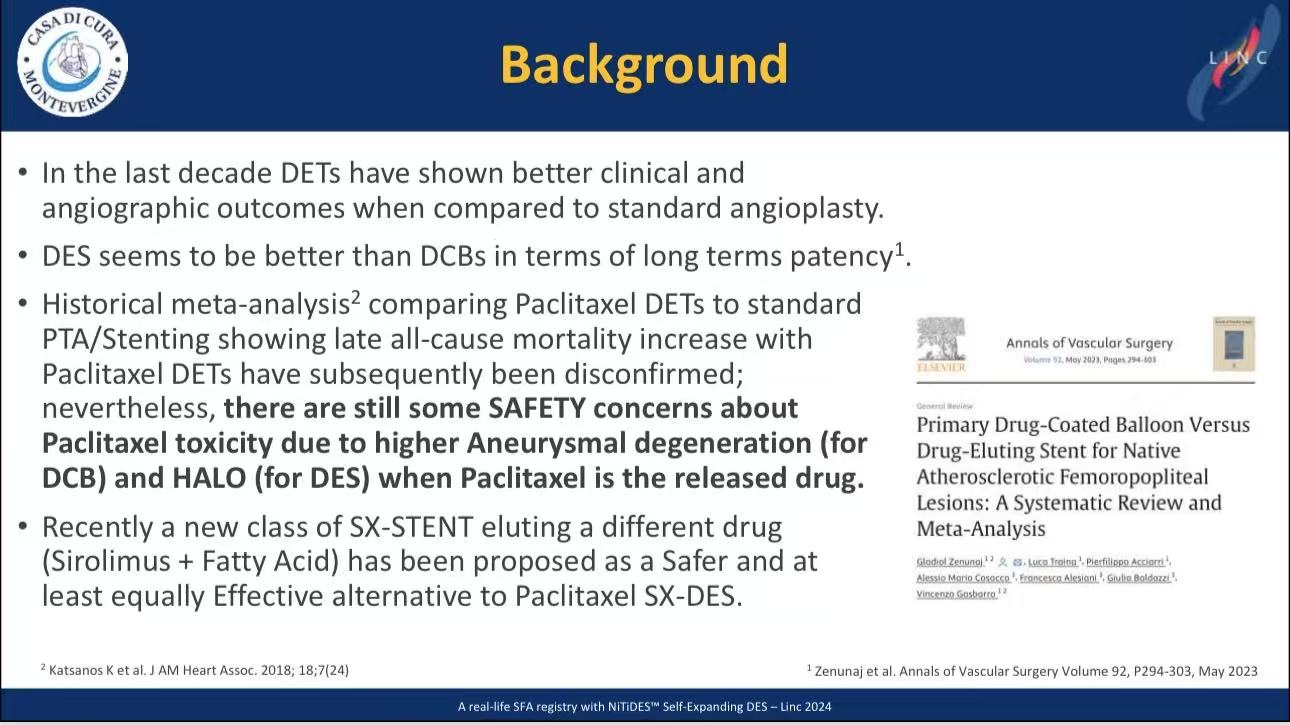
1. Patient Profile (n=77)
High-Risk Cohort: Diabetes (46.7%), dialysis (25%), Rutherford ≥3 (98.7%), CLI (48.1%)
Vs. ILLUMINA Trial: Longer lesions (151mm vs 72.5mm, p<0.001), severer stenosis (92.5% vs 84.3%, p<0.001)
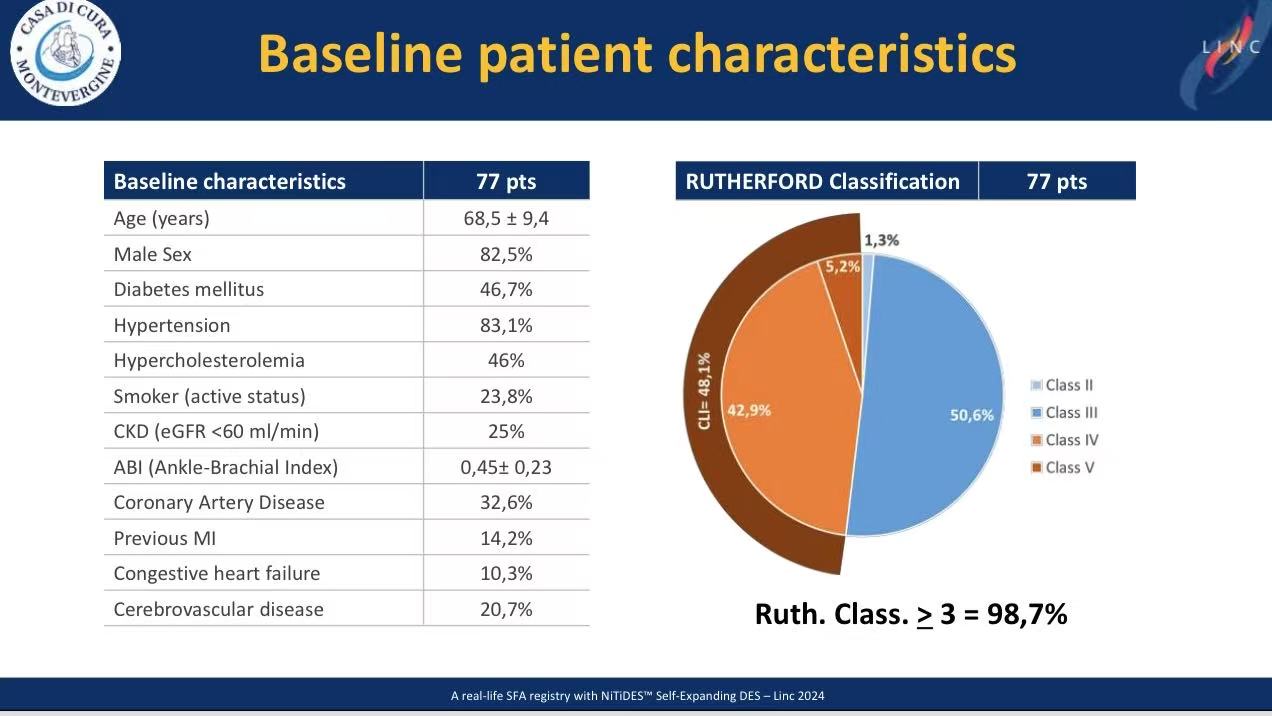
2. Lesion Complexity
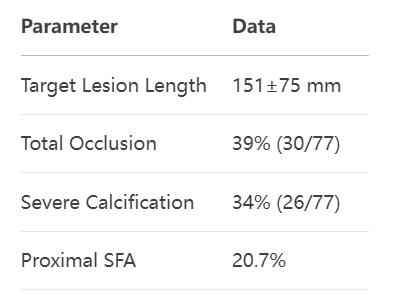
3. Technical Innovation
Retrograde Recanalization: For antegrade-failure CTO cases (case demonstration)
Precise Implantation: Abluminal reservoir technology ensures directional sirolimus delivery
4. Endpoints
Primary: 12-month MAE (all-cause death + amputation + CD-TLR)
Safety Core: HALO incidence (6/12/18/24-month duplex surveillance)
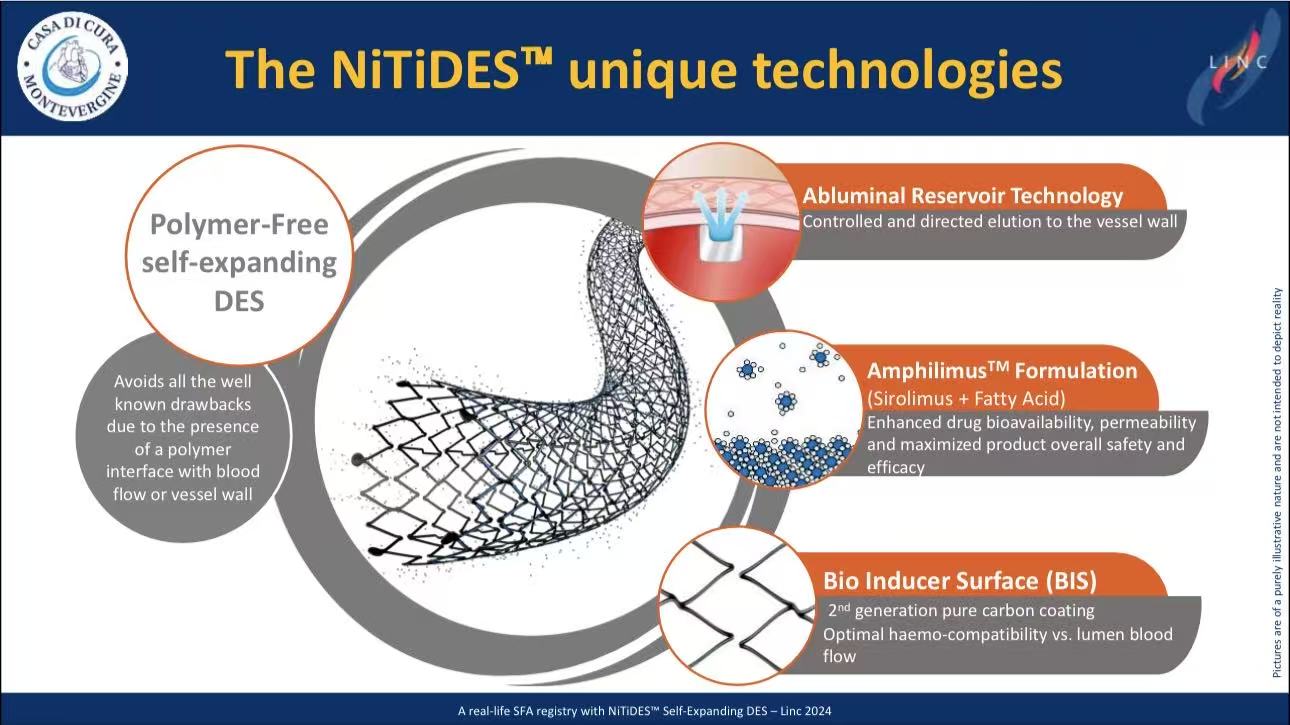
Technology Highlights: Why NITIDES™ for Complex Lesions?
Polymer-Free Platform
Eliminates polymer-induced inflammation, reducing late thrombosis risk.
Amphilimus™ Formulation
Sirolimus + fatty acid: 3× lower IC₅₀ vs. paclitaxel, enhancing bioavailability.
Abluminal Reservoir
Directional drug release sustained over 2-3 months (covers vascular repair phase).
Delivery Optimization
6F-compatible/0.035″ guidewire: Superior trackability (Reference: Medtronic Prevail DCB pushability 210gf).
Technical Note: While paclitaxel DCBs excel in pushability (e.g., Prevail™ 210gf), NITIDES™ combines mechanical support + controlled elution for diffuse lesions.
Conclusion: Future Directions
1.Safety First: Sirolimus devices mitigate paclitaxel toxicity in long/calcified/CLTI lesions.
2.Clinical Implications:
Prioritize retrograde access + directional drug delivery for CTOs.
Aggressive lesion preparation (IVL/atherectomy) for calcified segments.
3.Research Outlook: NITIDES™ 1-year MAE/HALO data at LINC 2025 will validate polymer-free DES efficacy.
Practice Guidance: For Rutherford 4-5 diffuse femoropopliteal disease, consider polymer-free DES + intravascular imaging to balance scaffolding and patency.



