Abstract
As the first prospective randomized three-arm study comparing drug-coated balloons (DCB), drug-eluting stents (DES), and bare-metal stents (BMS) for TASC C/D femoropopliteal lesions, the SPORTS trial demonstrated superior 12-month angiographic and clinical outcomes with DES (Eluvia™). DCB (SeQuent Please™) showed high bailout stenting rates (58%) impacting long-term patency. This article integrates device characteristics to guide complex lesion management.
Introduction
Treatment selection for TASC C/D femoropopliteal lesions has long lacked high-level evidence. Conventional interventions face challenges including high restenosis rates. The SPORTS trial, with randomized data from 224 patients (mean lesion length 22.3cm), provides critical decision-making insights.
Key Findings: SPORTS Trial Outcomes
1. Study Design
Three-arm RCT: 224 patients (Rutherford 2-4, lesions ≥13cm) randomized to:
DCB: SeQuent Please™ OTW (B.Braun)
DES: Eluvia™ (Boston Scientific)
BMS: Bare nitinol stent
Primary endpoint: Core lab-assessed angiographic stenosis at 12 months
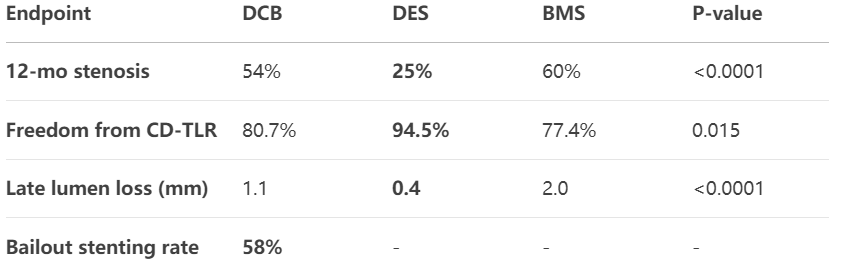
2. Critical Results
Subgroup analysis:
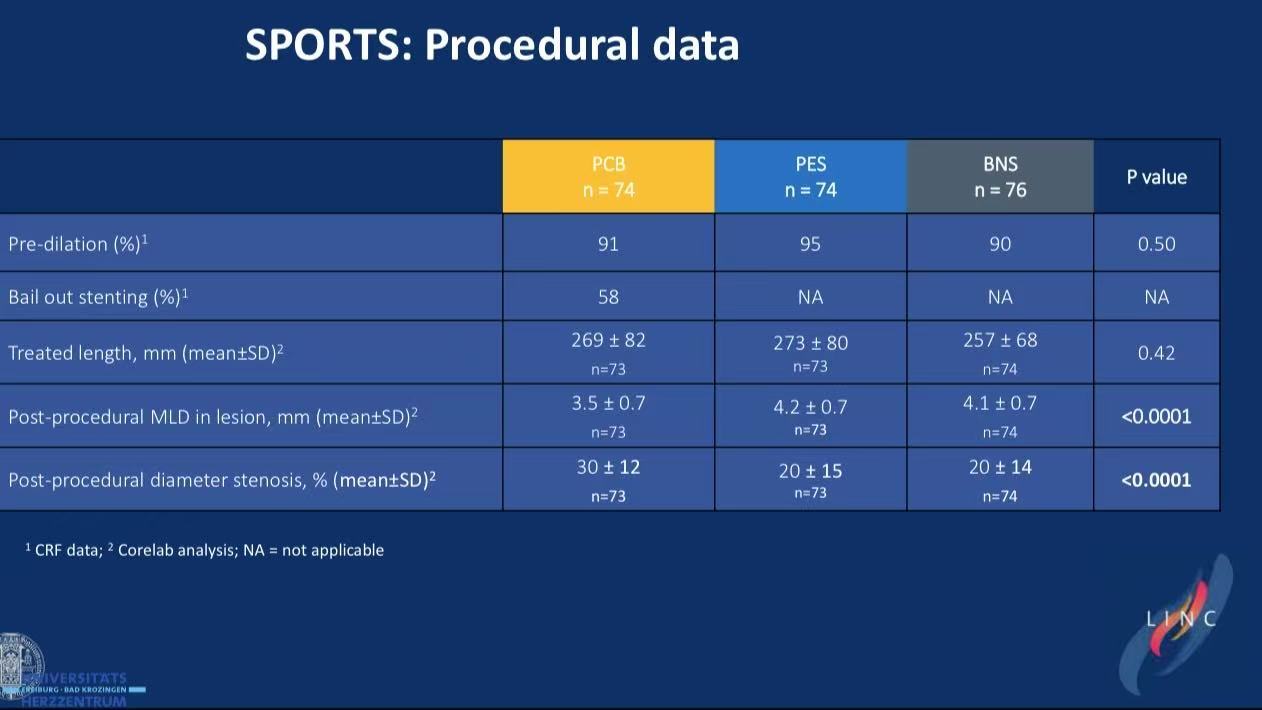
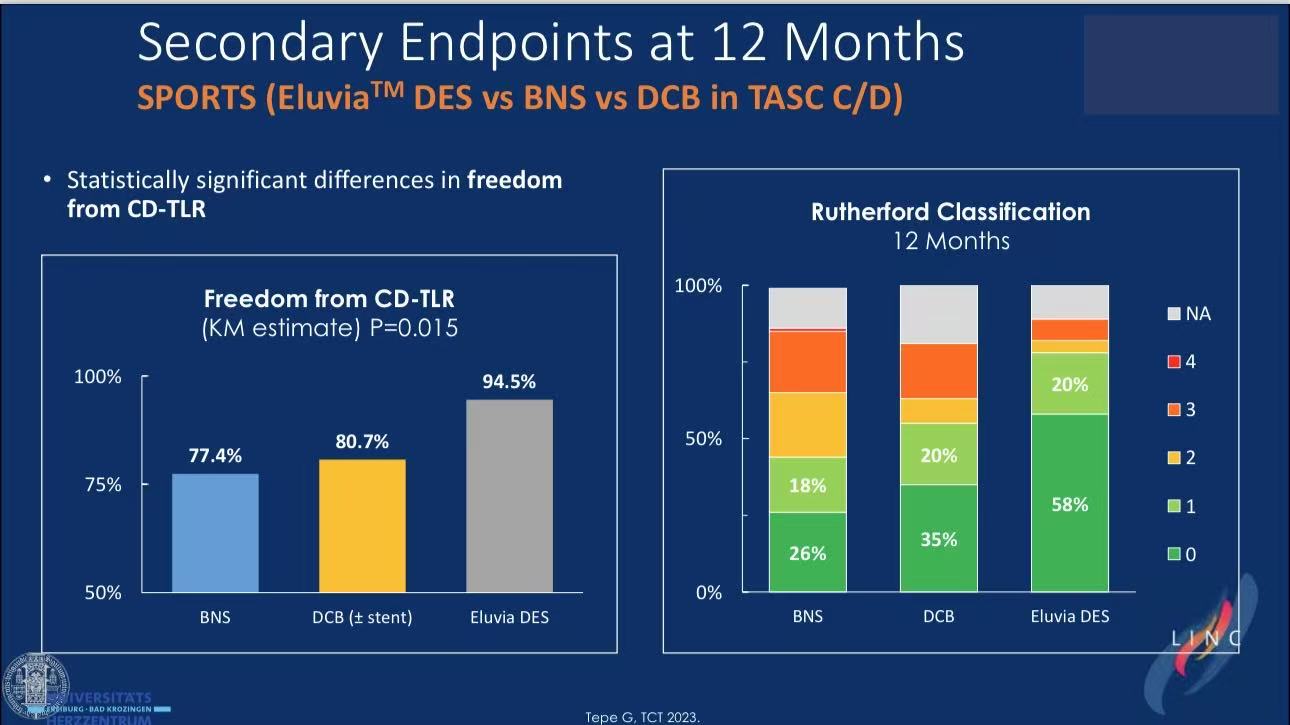
DCB bailout cause: 58% required stenting for dissections
Calcification impact: DES advantage amplified in moderate-severe calcification (38-41%)
3. Clinical Implications
DES superiority: Eluvia™ achieved 34.7% lower stenosis vs BMS in long lesions
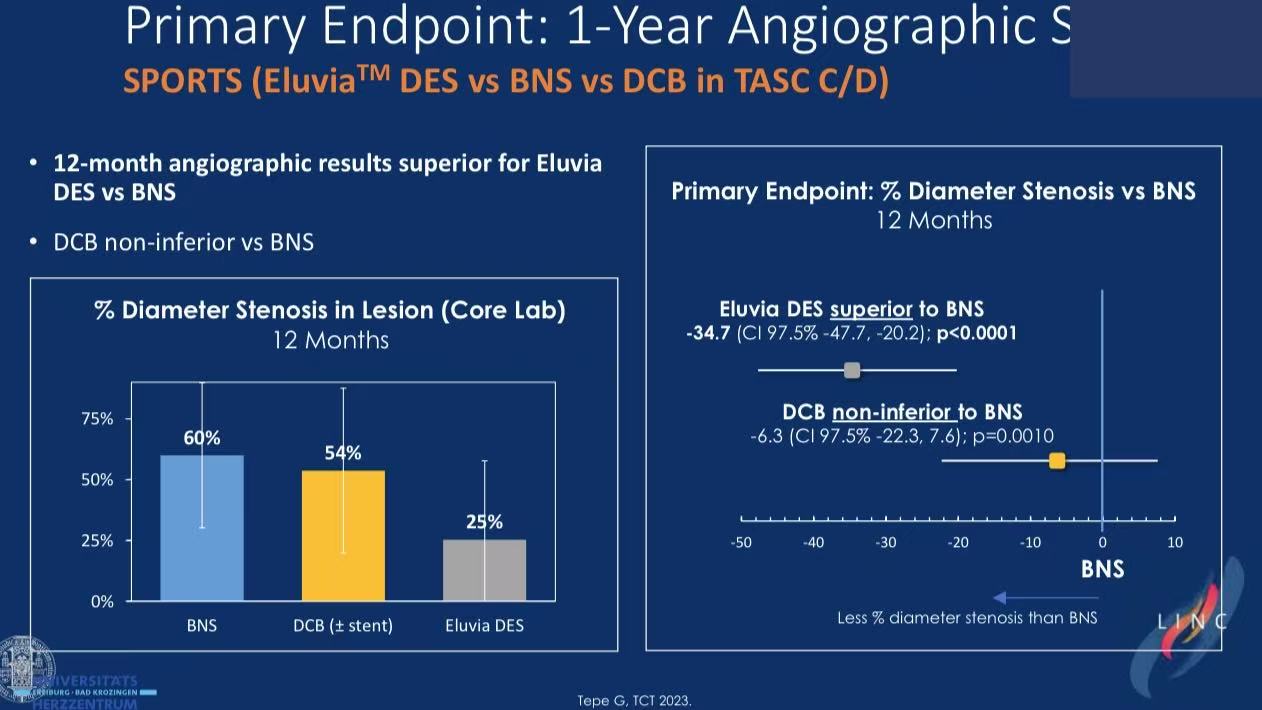
DCB limitations: SeQuent Please™ non-inferior to BMS but 60% required bailout
Lesion-specific: DES showed greater lumen gain in occlusions (MLD 4.2±0.7mm)
Technological Advances: DCB Optimization
Data from Medtronic's Prevail DCB highlight evolutionary directions:
Delivery enhancement:
Pushability: Next-gen DCBs reach 210g (vs 148g in SeQuent Please™ NEO)
Trackability: Tapered tip + ultrathin balloon material reduce resistance
Drug delivery:
Urea excipient: Rapid drug transfer in 30-60s (similar to SeQuent Please™)
Paclitaxel dose: 3.5μg/mm² (aligned with IN.PACT series)
Evidence base:
SCAAR study: 0.8% thrombosis rate at 2 years in 1,797 patients
Complex lesion efficacy: Strong performance in bifurcation (22.8%) and ISR (28%)
Conclusion
For TASC C/D lesions, SPORTS evidence supports:
First-line strategy: DES (Eluvia™) for lesions >15cm, especially calcified/occluded;
DCB role: SeQuent Please™ for stent-averse patients, with bailout stents available;
Technology evolution: Next-gen DCBs must overcome delivery limitations (e.g., Prevail's 210g pushability).
Future studies require >1-year data to validate DES durability and explore DCB-stent hybrid approaches.


