Presenter: Koen Deloose, MD
Affiliation: Department of Vascular Surgery, AZ Sint-Blasius, Dendermonde, Belgium
Abstract
The TINTIN trial, as the first prospective study evaluating Luminor drug-coated balloon (DCB) combined with iVolution stent for TASC C/D femoropopliteal lesions, demonstrated 62.5% 5-year freedom from clinically driven target lesion revascularization (CD-TLR) in lesions averaging 24cm (60% CTOs, 73% moderate-severe calcification). This analysis integrates technical features of the newly approved Luminor 18 RX in Japan to provide high-level evidence for complex lower extremity arterial disease management.
Introduction
TASC C/D femoropopliteal lesions remain a therapeutic challenge in vascular surgery. Conventional interventions face high restenosis rates and suboptimal long-term patency. The 5-year results of Belgium's TINTIN trial and the recent Japanese approval of iVascular's Luminor DCB offer innovative solutions. This article elucidates the technical rationale and clinical value of this combined strategy.
Key Findings: TINTIN Trial Outcomes
1. Study Design
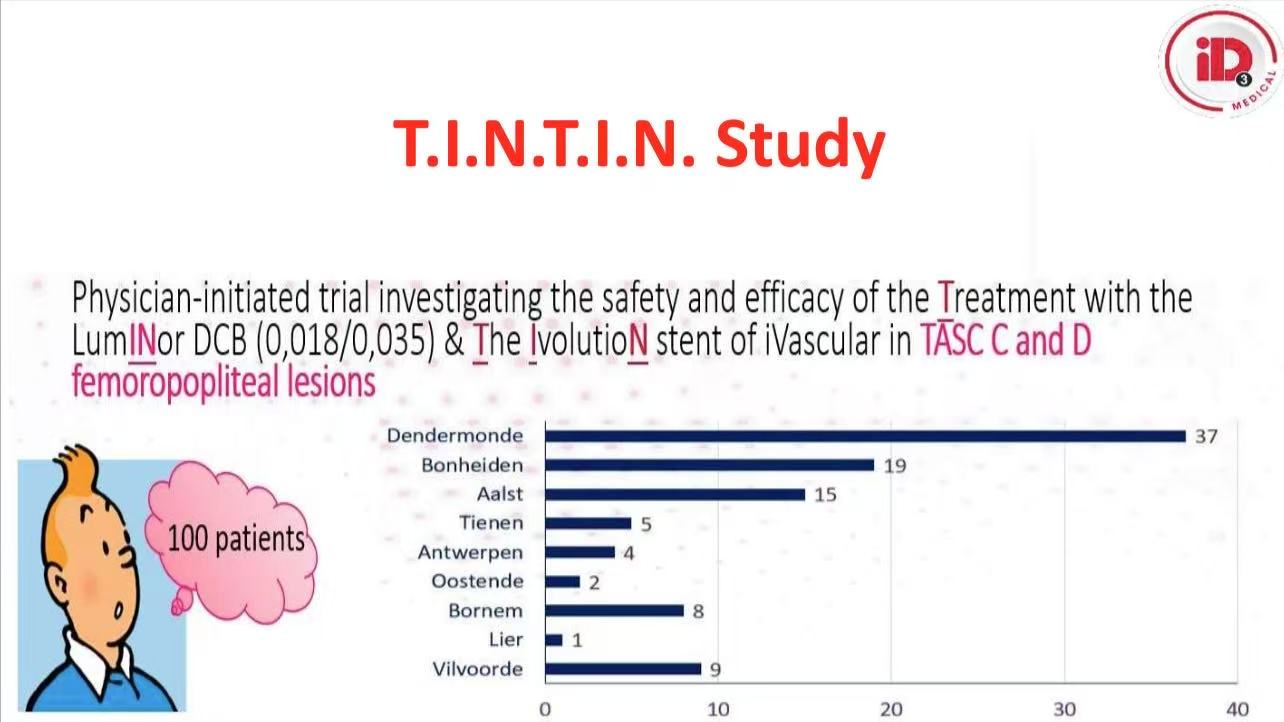
Prospective multicenter trial: 100 patients with TASC C/D lesions (mean length 242.65mm, 60% CTO, 73% moderate-severe calcification)
Protocol: Luminor DCB (2.5μg/mm² paclitaxel) + iVolution stent implantation
Primary endpoint: 12-month freedom from CD-TLR
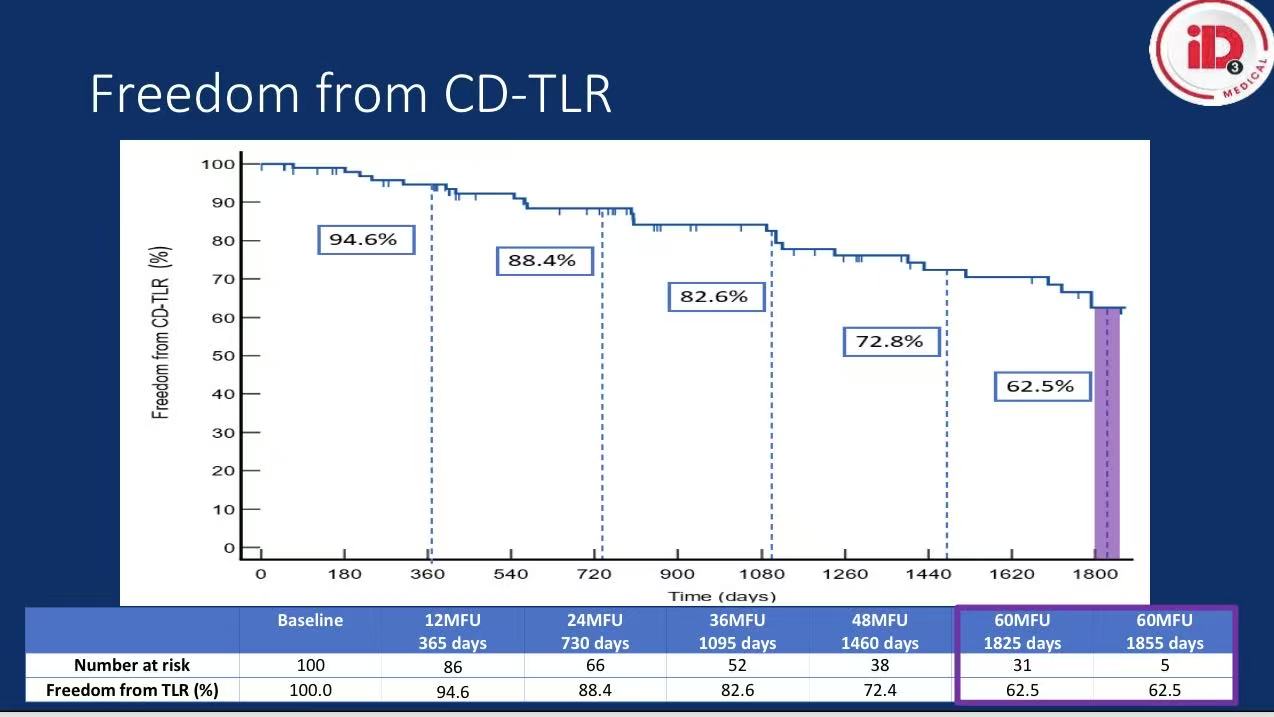
2. Critical Results
Efficacy:
1-year freedom from CD-TLR: 88.4%
5-year freedom from CD-TLR: 62.5%, superior to POBA (73.7%, P=0.0001)
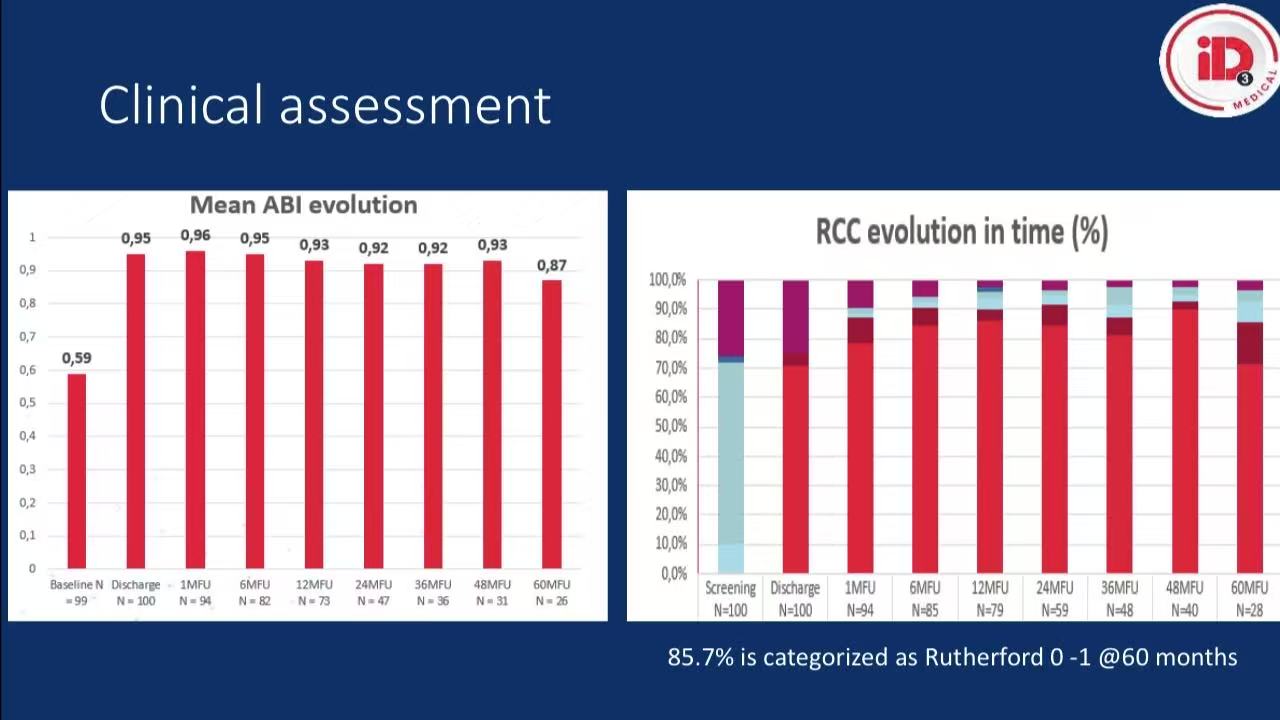
Clinical improvement: 85.7% achieved Rutherford 0-1 at 5 years
Lesion-specific outcomes:
For >20cm lesions: 54.5% 5-year CD-TLR rate
15% reduced patency in severe calcification
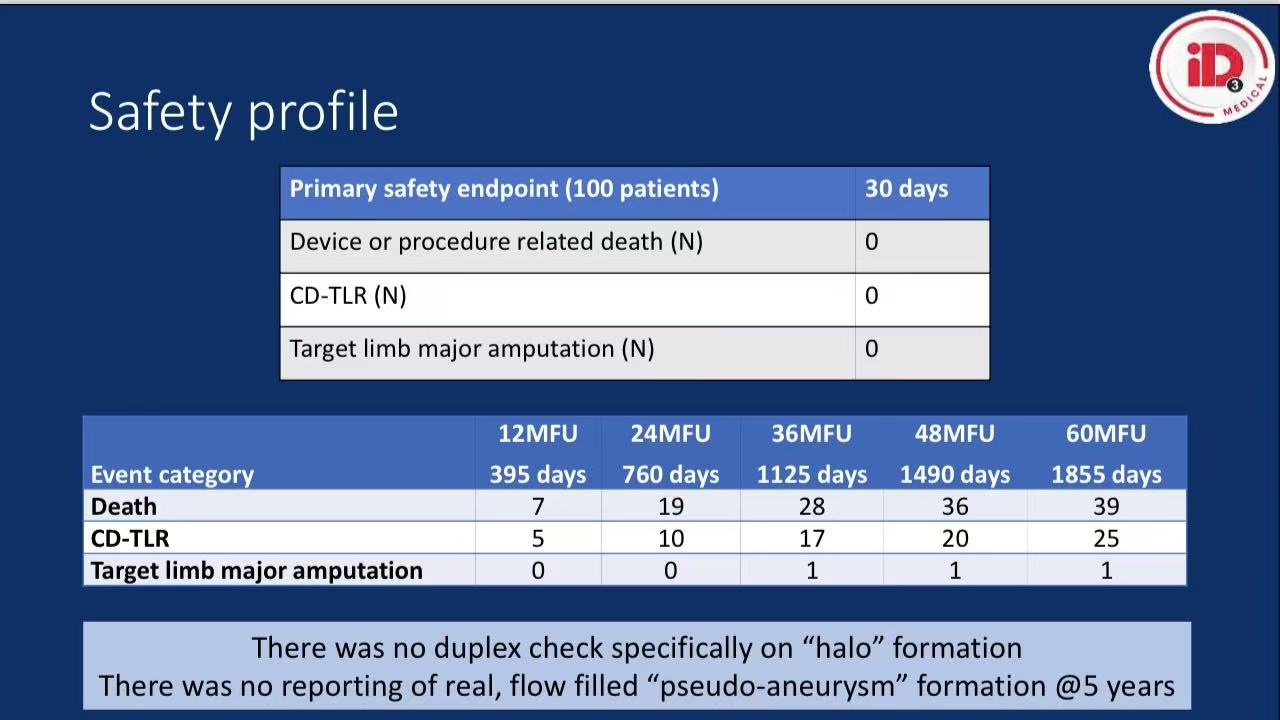
Safety:
30-day MAEs: 0% (no death/major amputation)
5-year survival: 57.5% (consistent with CLTI baseline prognosis)
3. Technical Insights
Combination advantage: DCB inhibits hyperplasia + stent provides scaffolding
Procedural details: 88% required predilation, mean 1.82 Luminor balloons used
Vessel preparation: Recommended 5.74mm stent post-dilation for 5.5mm reference
Technological Advancements: Luminor DCB
The Japan-approved Luminor 18 RX features:
Enhanced drug delivery:
1.2.5μg/mm² paclitaxel: Balanced efficacy-safety profile
2.Composite excipients: Urea + polysorbate sorbitol for tissue retention
3.Crystalline/amorphous mix: Rapid release + sustained effect
Delivery optimization:
Oceanus platform: Superior navigation in tortuosity (99.2% success in Japan SOL trial)
18RX system: Designed for Asian anatomy
Evidence base:
Global studies: 82.1% TLR-free in >15cm lesions
5-year freedom from aneurysmal degeneration
Conclusion
For complex femoropopliteal disease, TINTIN 5-year data indicate:
1.Combination therapy: Luminor DCB + iVolution stent is reliable for TASC C/D;
2.Technical keys: Adequate predilation + stent post-dilation optimize outcomes;
3.Patient selection: CLTI requires balancing revascularization with prognosis.
With Luminor DCB's expansion in Asia, its optimized delivery system will offer safer treatment for anatomically complex patients.


