Presenter: Sabine Steiner, MD
Affiliation: Division of Angiology, University Hospital Leipzig; Helmholtz Institute for Metabolism, Obesity and Vascular Research, Germany
Abstract
As the first head-to-head randomized trial comparing different paclitaxel doses in drug-coated balloons (DCBs), the COMPARE trial's 5-year follow-up demonstrated comparable efficacy between low-dose Ranger™ DCB (2.0μg/mm²) and high-dose IN.PACT Admiral™ DCB (3.5μg/mm²) for complex femoropopliteal lesions. This article integrates the technical advantages of Medtronic's next-generation IN.PACT Admiral™ XL to provide evidence-based guidance for managing long-segment calcified disease.
Introduction
In peripheral artery disease (PAD) treatment, DCBs have become first-line interventions for femoropopliteal lesions. However, debates persist regarding optimal paclitaxel dosing and coating technologies. The 2024 5-year COMPARE trial data and China's approval of Medtronic's IN.PACT Admiral™ XL (200/250mm) offer new solutions for complex lesions. This analysis synthesizes key clinical evidence and technological advancements.

Key Findings: COMPARE Trial 5-Year Outcomes
1. Study Design
Multicenter RCT: 414 patients with femoropopliteal lesions (40.6%-43% CTOs, 50.5%-57.1% moderate-severe calcification) randomized to:
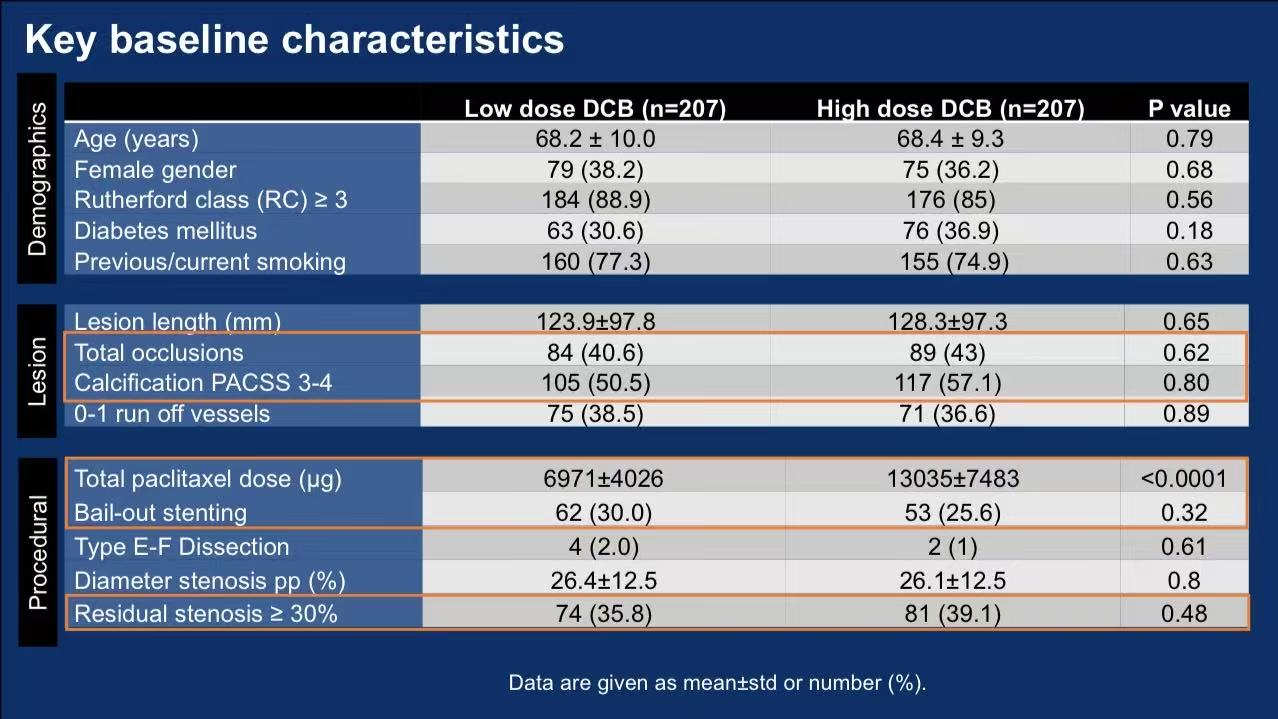
Low-dose: Ranger™ DCB (2.0μg/mm², citrate ester excipient)
High-dose: IN.PACT Admiral™/Pacific™ DCB (3.5μg/mm², urea excipient)
Primary endpoint: 12-month primary patency (non-inferiority design)
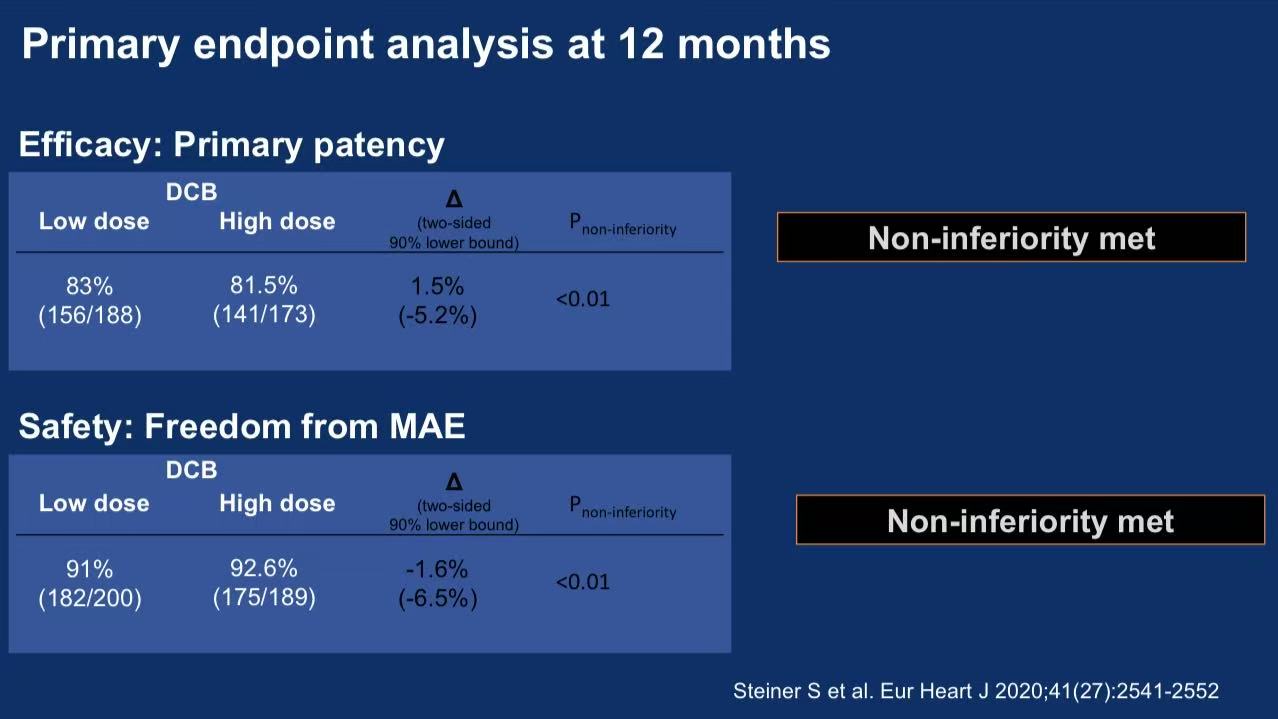
2. Critical Results
Efficacy:
12-month patency: 83% (low-dose) vs 81.5% (high-dose) (Δ=1.5%, P<0.01 for non-inferiority)
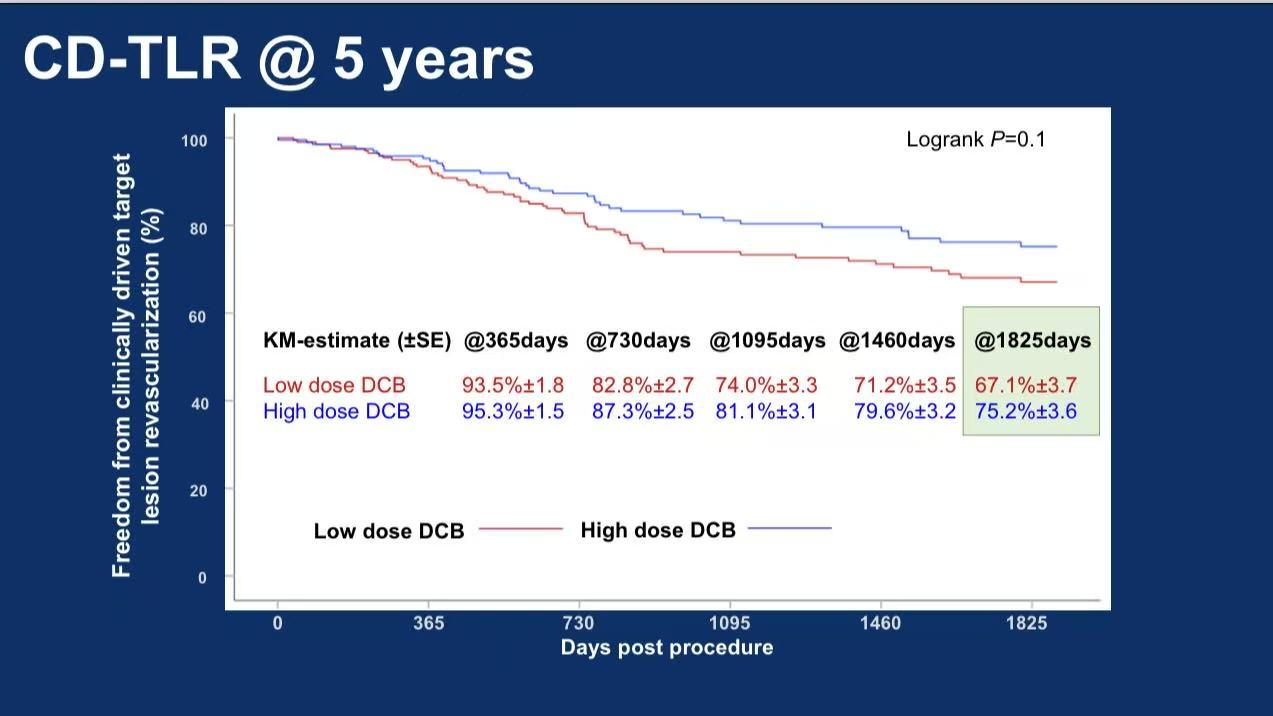
5-year freedom from CD-TLR: 67.1% vs 75.2% (P=0.1)
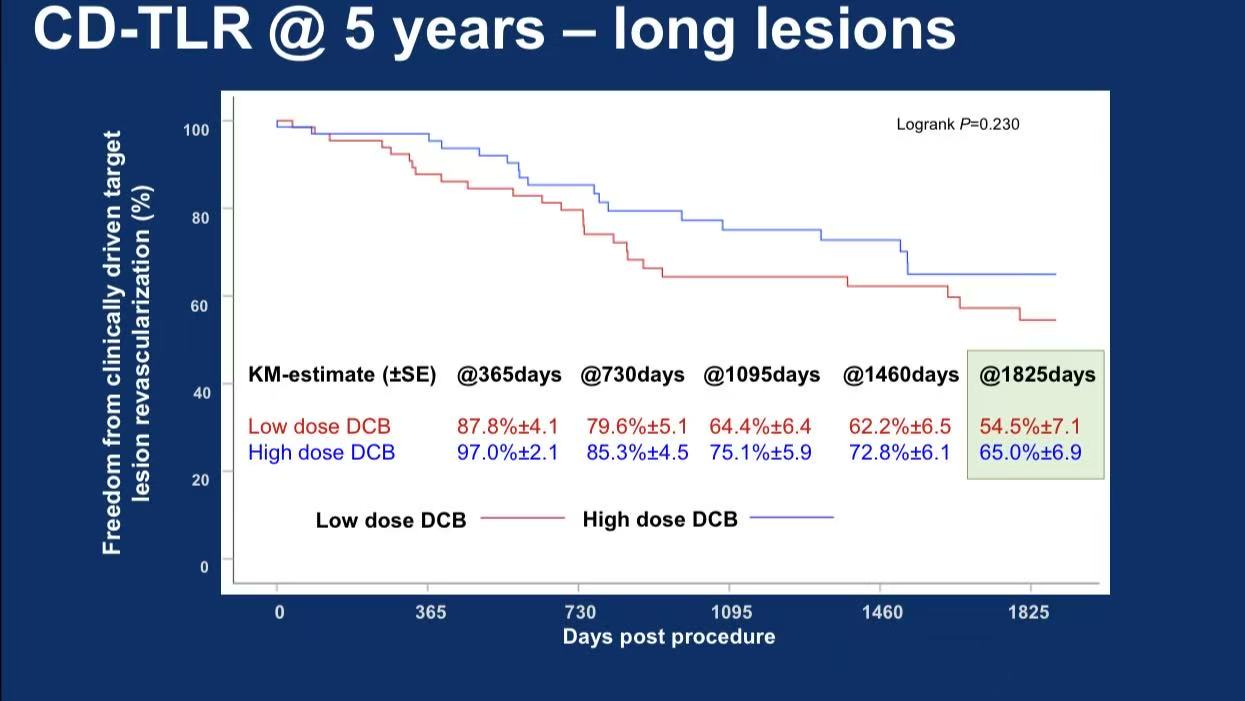
Subgroup: High-dose DCB showed superior 5-year CD-TLR in long lesions (>20cm): 65.0% vs 54.5% (P=0.230)
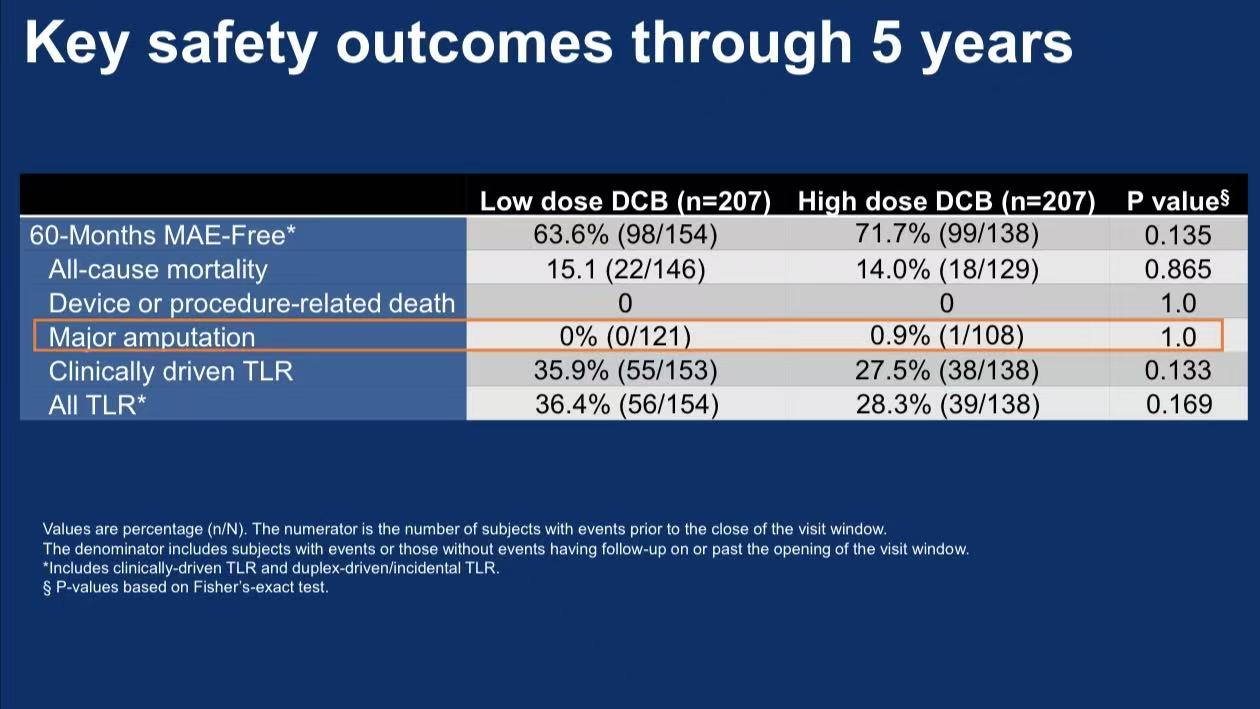
Safety:
5-year all-cause mortality: 15.1% vs 14.0% (P=0.865)
Major amputation: 0.9% in high-dose group, no dose-dependent risk
3. Clinical Implications
Dosing: High-dose DCBs (e.g., IN.PACT Admiral™) may optimize outcomes in long/calcified lesions.
Technique: 58% bailout ste
Technological Advancements: IN.PACT Admiral™ XL
Medtronic's newly approved long-balloon addresses COMPARE trial challenges:
Extended Lesion Coverage:
250mm single-balloon length matches mean lesion length in COMPARE (123.9-128.3mm), minimizing overlap.
Enhanced Drug Delivery:
3.5μg/mm² paclitaxel: COMPARE confirmed superiority in long lesions
Urea excipient: Hydrophilic coating enables 180-day sustained release (30% higher tissue concentration vs hydrophobic coatings)
Evidence Base:
IN.PACT SFA 5-year data: 74.5% freedom from CD-TLR vs 65.3% with PTA (P<0.01)
500,000+ global cases with 91% patency in calcified lesions

Conclusion
For complex femoropopliteal disease, COMPARE 5-year data support:
Dosing: High-dose (3.5μg/mm²) DCBs for long/calcified lesions;
Technology: IN.PACT Admiral™ XL optimizes procedural efficiency;
Strategy: DCB+bailout stenting with intravascular imaging emerges as first-line for TASC C/D lesions.
With growing adoption of "implant-free" interventions, DCB innovations will continue improving long-term PAD outcomes.



