Yoshimitsu Soga, MD, PhD
Kokura Memorial Hospital, Fukuoka, Japan
Coordinating Investigator of SOL Japan Trial
Abstract
The SOL Japan trial demonstrated outstanding efficacy of MEDICO'S HIRATA's Luminor drug-coated balloon (DCB) in treating femoropopliteal artery lesions in Japanese population: 94.9% primary patency rate and 99.2% freedom from target lesion revascularization (TLR) at 12 months. This prospective multicenter study enrolled 122 patients, providing crucial evidence for DCB therapy in Asian population.
Introduction
With accelerating population aging, the prevalence of femoropopliteal artery disease continues to rise in Asia. However, conventional percutaneous transluminal angioplasty (PTA) shows limited efficacy in long-segment and calcified lesions. The Luminor DCB, with its innovative drug delivery system and optimized balloon platform, has delivered impressive results in the SOL Japan trial, offering a new therapeutic option for East Asian patients with peripheral artery disease.
Study Content and Results
The SOL Japan trial is a prospective, multicenter, single-arm study conducted across 12 Japanese institutions, primarily evaluating the safety and efficacy of Luminor DCB for femoropopliteal artery lesions.

Study Design Highlights
Stringent Patient Selection:
Inclusion: Rutherford 2-4, native SFA/proximal PA lesions (≤200mm), successful predilation
Exclusion: In-stent restenosis, PTA-resistant severe calcification
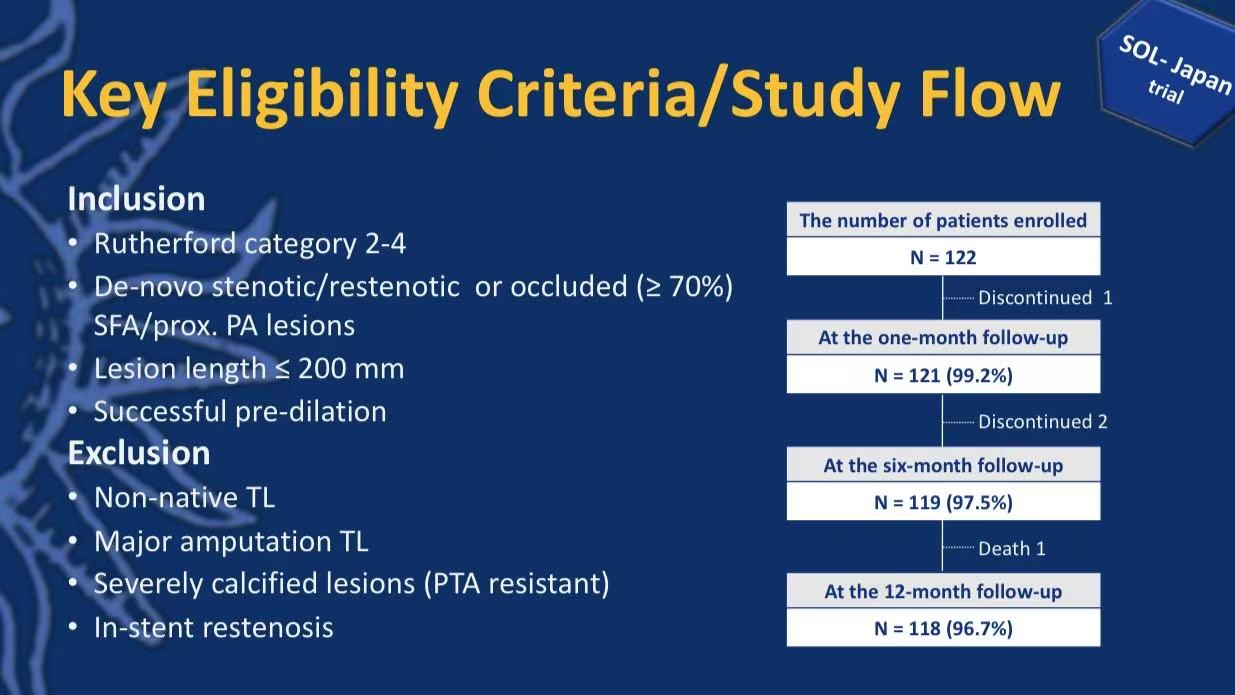
Study Population Characteristics:
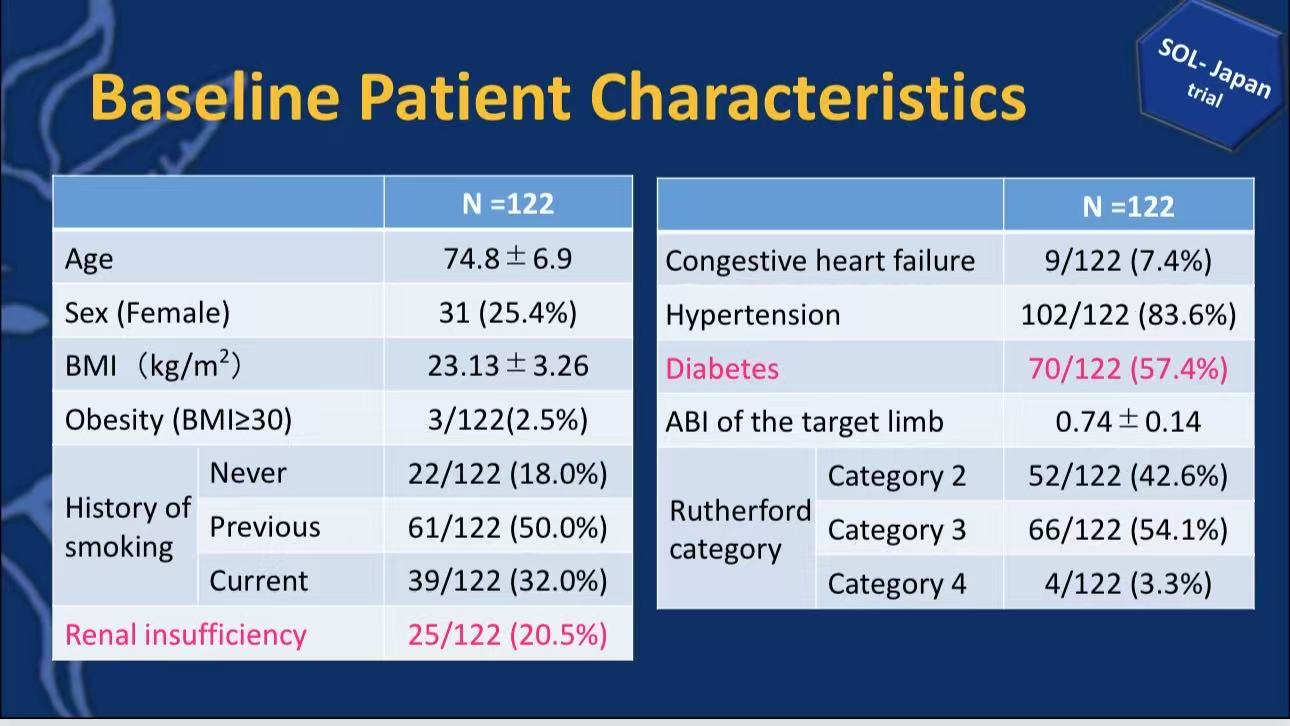
Mean age 74.8 years, 25.4% female
High comorbidity burden: diabetes 57.4%, hypertension 83.6%, smoking history 82.0%
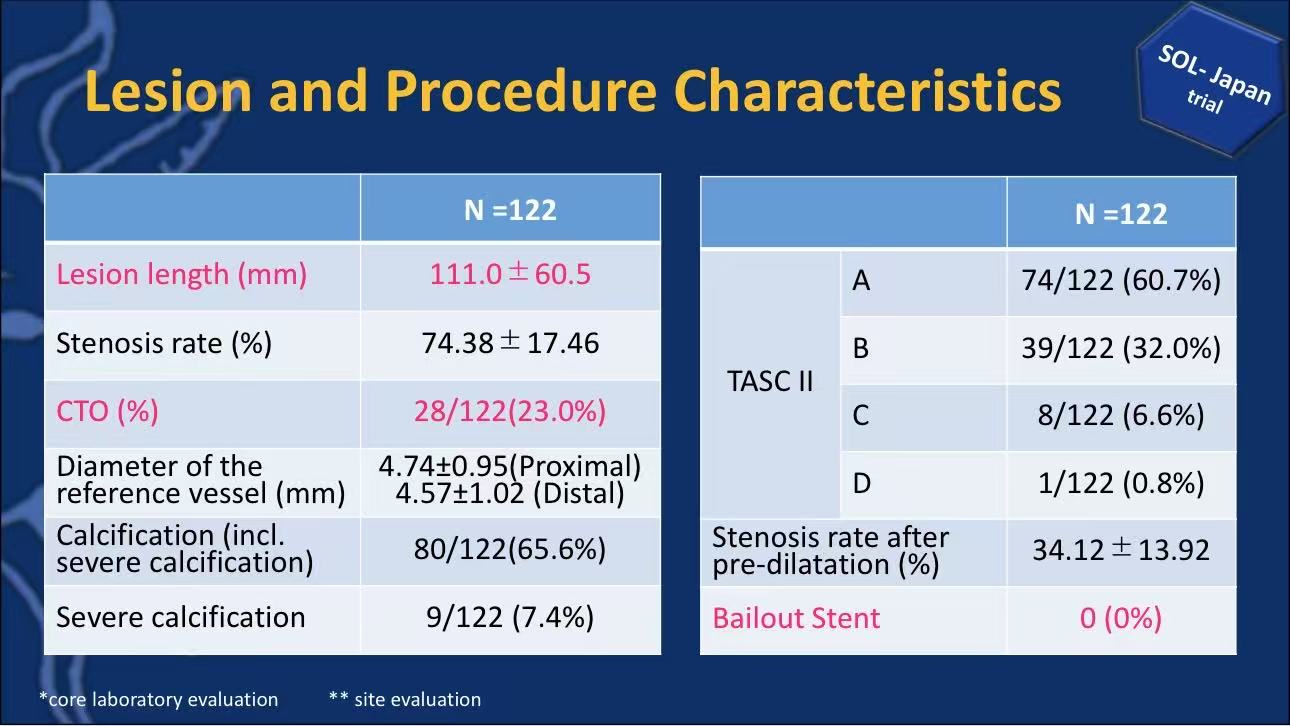
Lesion features: mean length 111.0±60.5mm, 23.0% CTO, 65.6% calcification
Key Efficacy Outcomes
Imaging Assessment:
12-month primary patency (PSVR≤2.4) reached 94.9%
Residual stenosis reduced from 74.38% to 34.12% post-predilation
Clinical Endpoints:
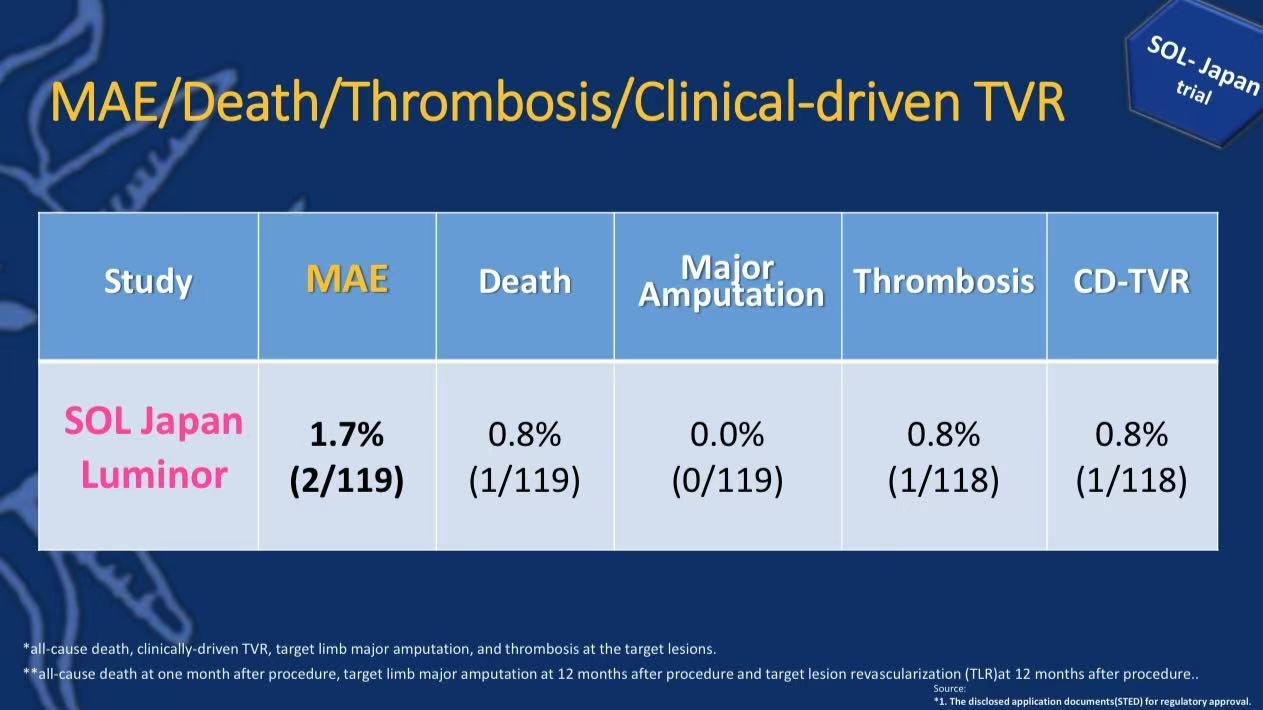
12-month freedom from CD-TLR 99.2%
Only 0.8% required target vessel revascularization
No major amputations occurred
Safety Profile:
Major adverse events (MAE) rate 1.7%
All-cause mortality 0.8%, target lesion thrombosis 0.8%
Subgroup Analysis Value
Consistent efficacy in long lesions (>100mm) and calcified lesions (65.6%)
Benefits maintained in TASC C/D complex lesions (7.4%)
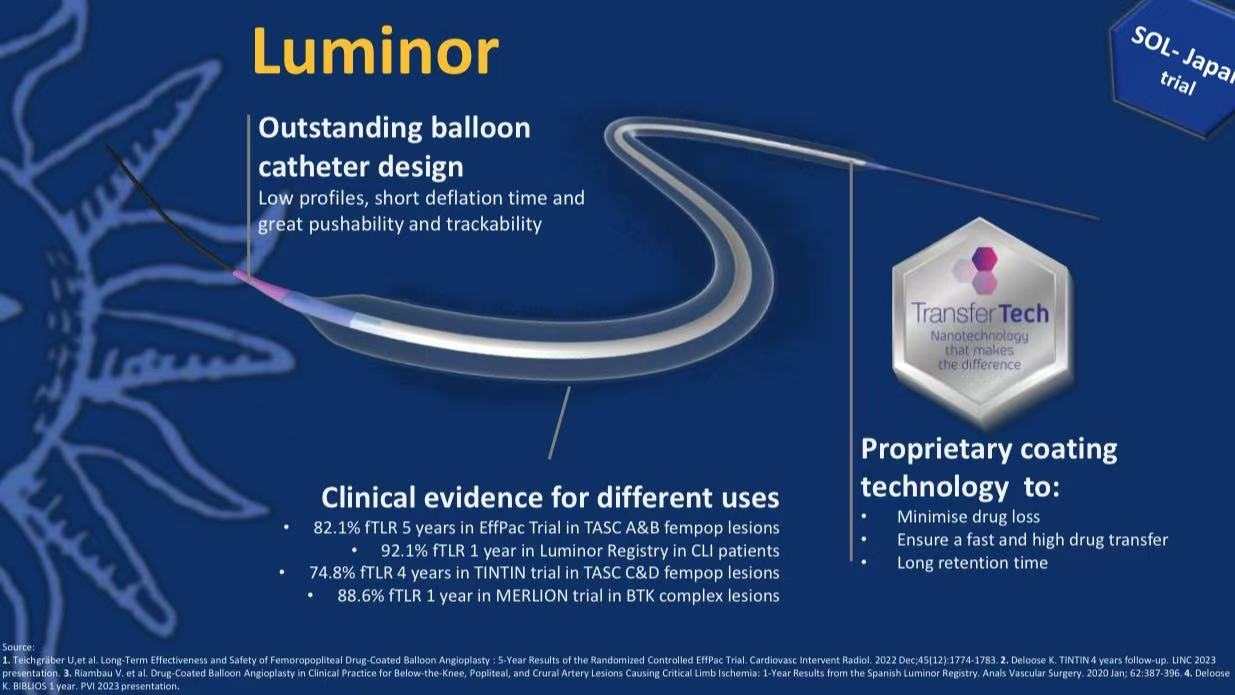
Product Technical Highlights
Combined with Japanese market approval information, Luminor DCB features:
Platform Advantages:
Low-profile design, short deflation time
Excellent pushability and trackability
Drug System Innovation:
Proprietary Transfer Tech nano-coating
Optimized excipient combination (urea-based)
Ensures rapid drug transfer and prolonged retention
Clinical Validation:
Supported by global studies (EfPac, TINTIN, MERLION)
Population-specific data for Asians
Conclusions and Clinical Implications
1.Luminor DCB provides a safe and effective option for Japanese/Asian population, filling regional evidence gaps
2.Excellent performance in complex anatomies (long lesions, calcification) expands DCB applications
3.Ultra-high TLR-free rate (99.2%) may shift current treatment paradigms
4.Design specifically considers Asian vascular characteristics
These findings will directly influence peripheral artery disease treatment guidelines in Asia-Pacific region, offering critical references for clinical practice.



