Marianne Brodmann, MD
Medical University of Graz, Austria
On behalf of the RANGER II SFA Investigators
Abstract
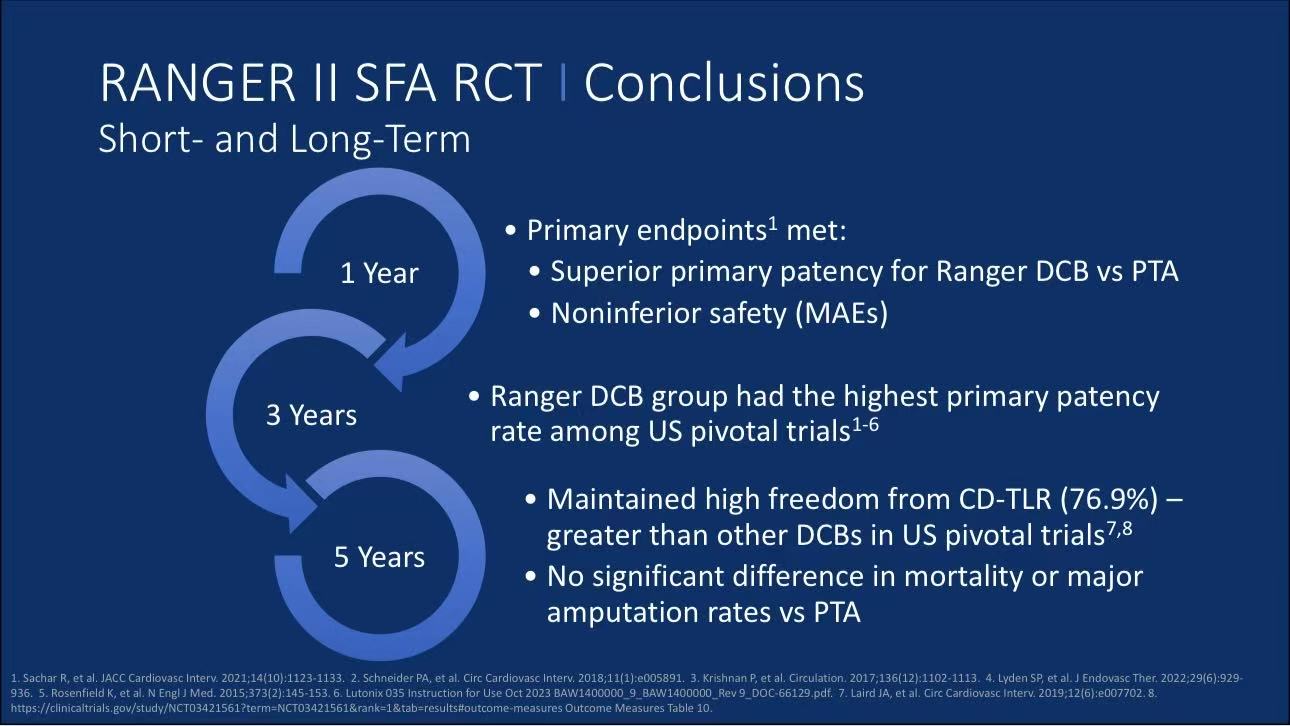
The 5-year follow-up data from the RANGER II SFA randomized controlled trial demonstrates outstanding long-term efficacy of Boston Scientific's Ranger™ drug-coated balloon (DCB) in treating femoropopliteal artery disease: 76.9% freedom from clinically-driven target lesion revascularization (CD-TLR), significantly superior to conventional PTA (74.8%), with no statistical differences in all-cause mortality or target limb major amputation rates. This study provides high-level evidence for the long-term safety and efficacy of DCB in complex peripheral artery disease.
Introduction
The most significant advancement in femoropopliteal artery disease treatment in recent years has been the application of drug-coating technology. However, controversies persist regarding long-term efficacy comparisons between different DCB products and safety concerns. The RANGER II SFA study, as an international multicenter randomized controlled trial, through rigorous 5-year follow-up, provides clinicians with long-term performance data of Ranger™ DCB in real-world complex lesions, offering critical guidance for treatment strategy selection.
Study Content and Results
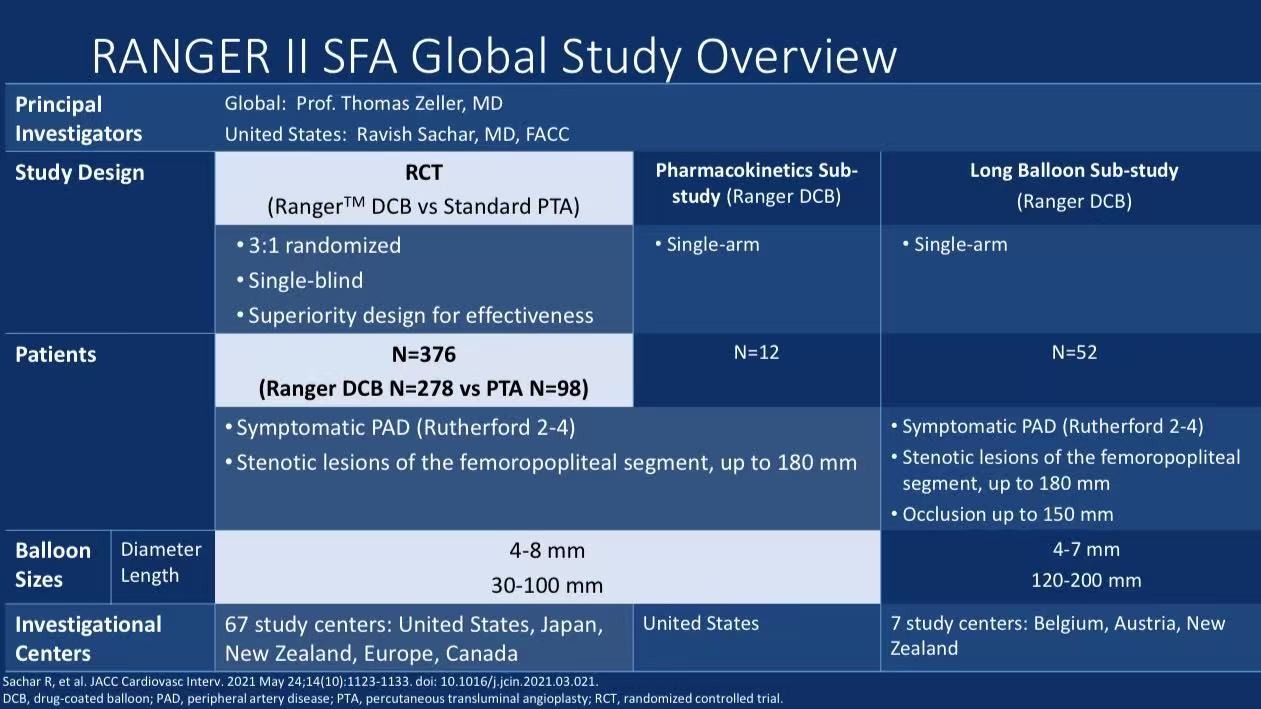
The RANGER II SFA study enrolled 376 patients with symptomatic peripheral artery disease (Rutherford 2-4), randomized 3:1 to Ranger™ DCB group (278 cases) or standard PTA group (98 cases). Key study design features:
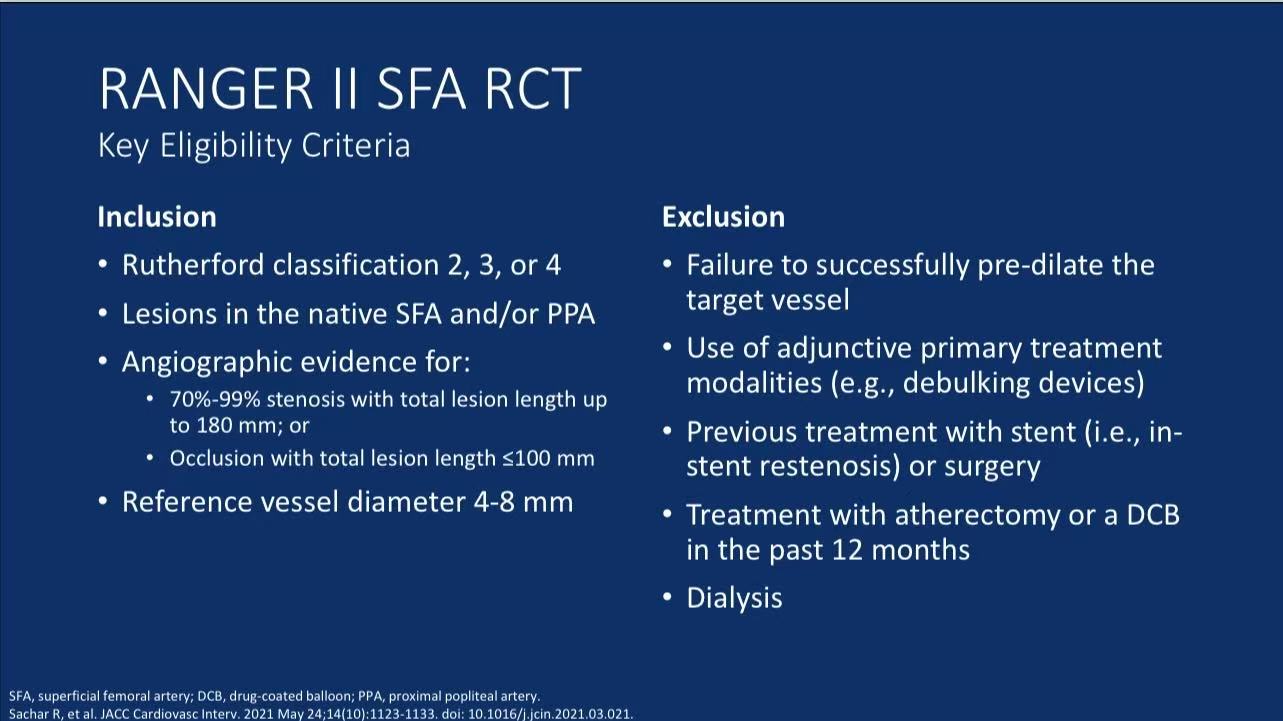
Stringent Patient Selection:
Inclusion: Native superficial femoral/proximal popliteal artery lesions ≤180mm (occlusions ≤100mm), reference vessel diameter 4-8mm
Exclusion: In-stent restenosis, DCB or atherectomy within 12 months, dialysis patients
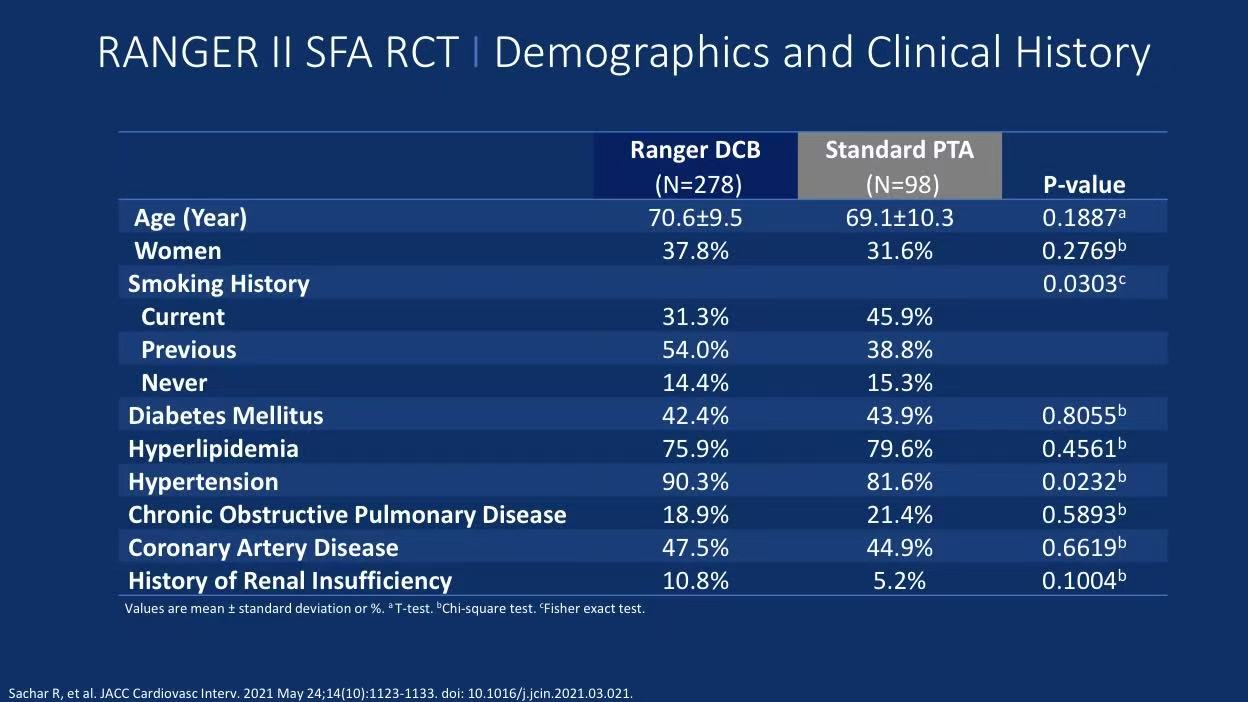
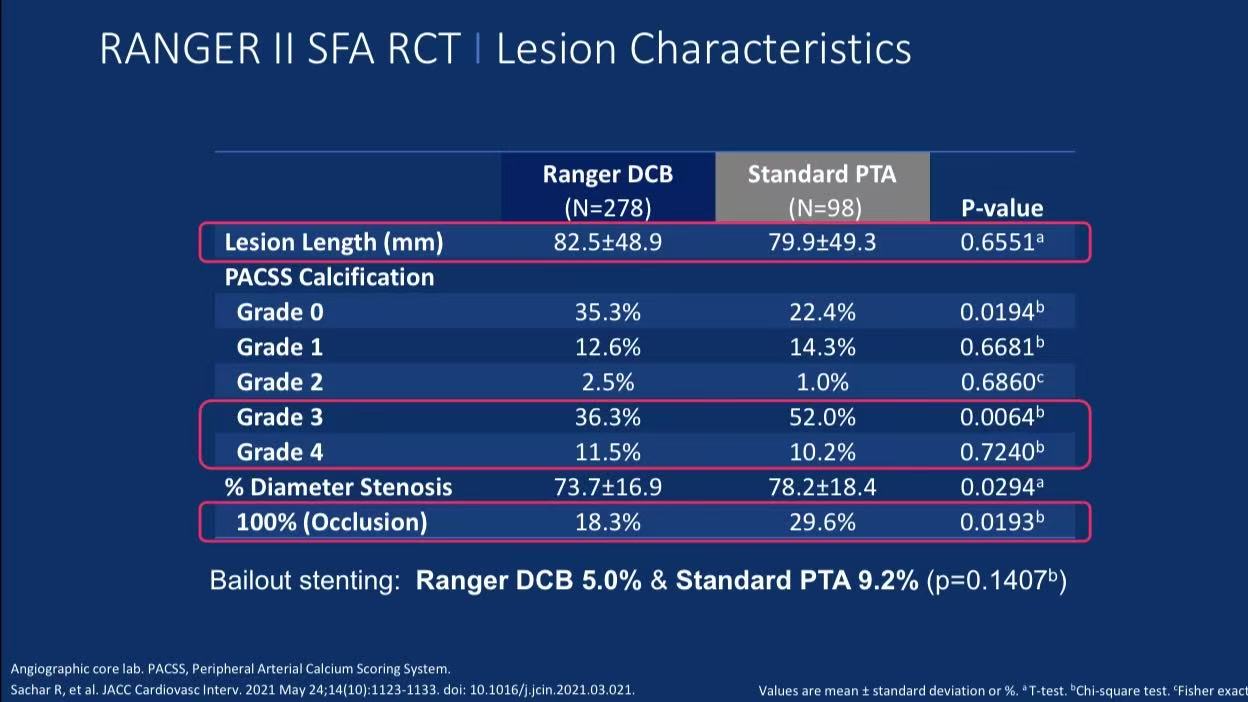
Lesion Characteristics:
Mean lesion length 82.5±48.9mm
18.3% total occlusions
47.8% moderate-severe calcification (PACSS 2-4)
Bailout stenting rate only 5.0% (significantly lower than 9.2% in PTA group)
Key Long-term Efficacy Findings:
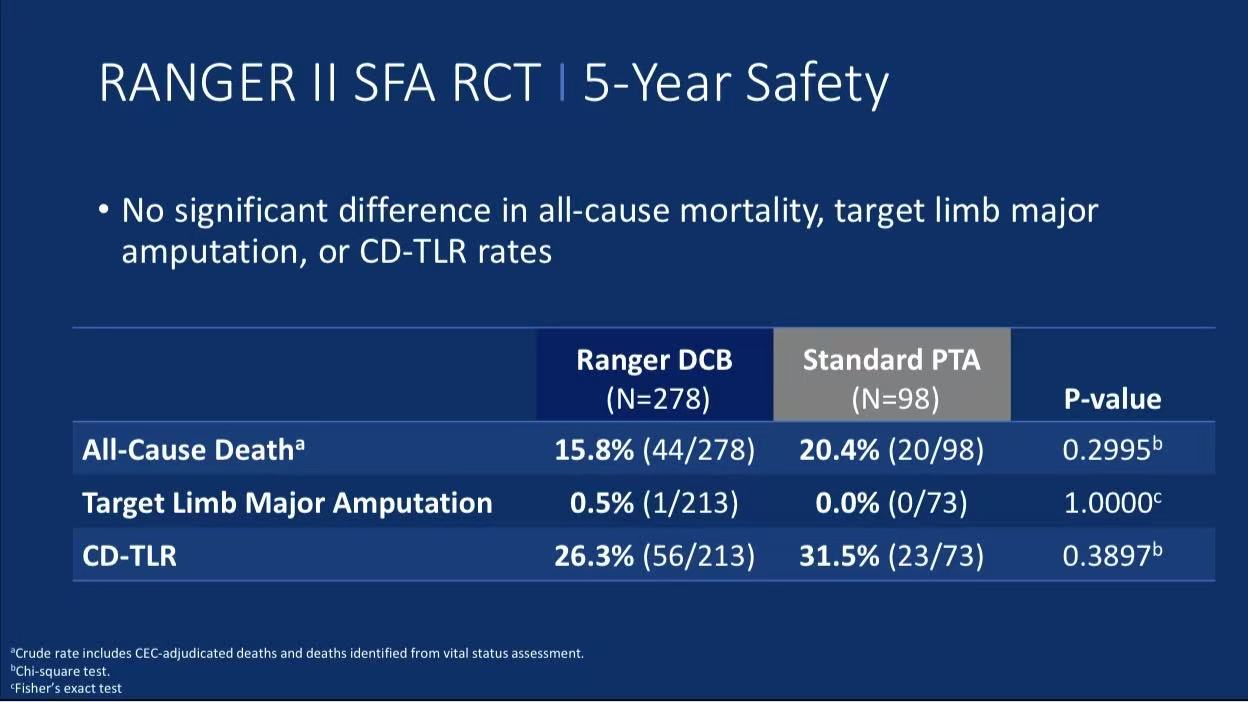
CD-TLR Rates: 26.3% in Ranger™ DCB group vs 31.5% in PTA group at 5 years (p=0.3897)
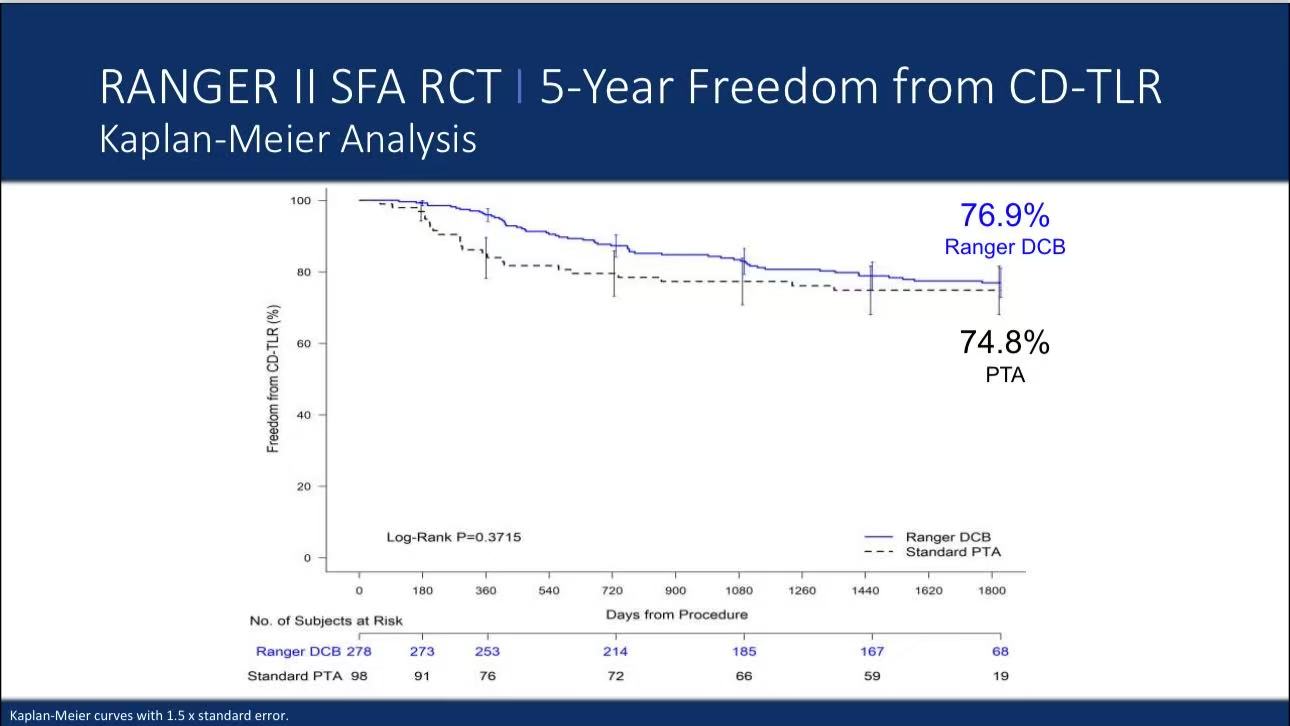
Survival Analysis: Kaplan-Meier curves showed 76.9% freedom from CD-TLR in DCB group vs 74.8% in PTA group (Log-Rank p=0.3715)
Safety Endpoints: No significant differences in all-cause mortality (15.8% vs 20.4%, p=0.2995) or target limb major amputation (0.5% vs 0%)
Subgroup Analysis Value:
Consistent efficacy in high-risk subgroups (diabetes 42.4%, severe calcification 11.5%)
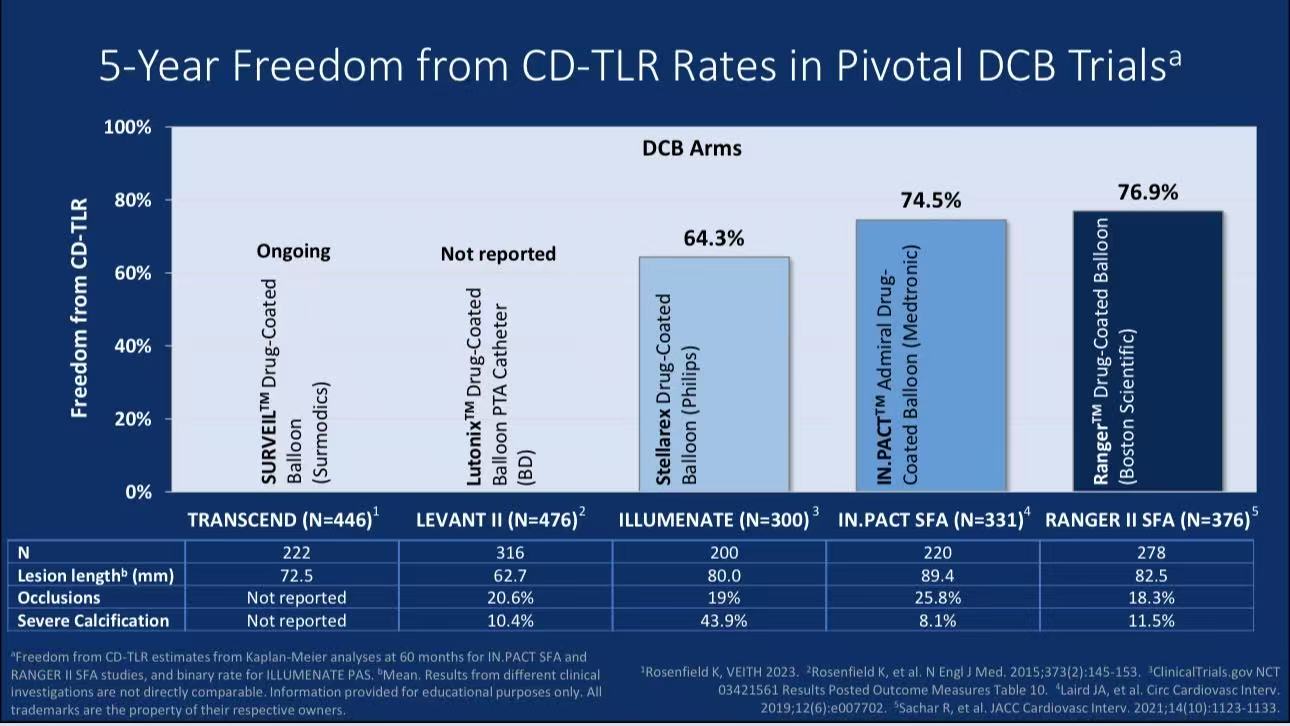
Compared with other mainstream DCBs (IN.PACT Admiral, Stellarex), Ranger™ DCB showed superior 5-year freedom from CD-TLR
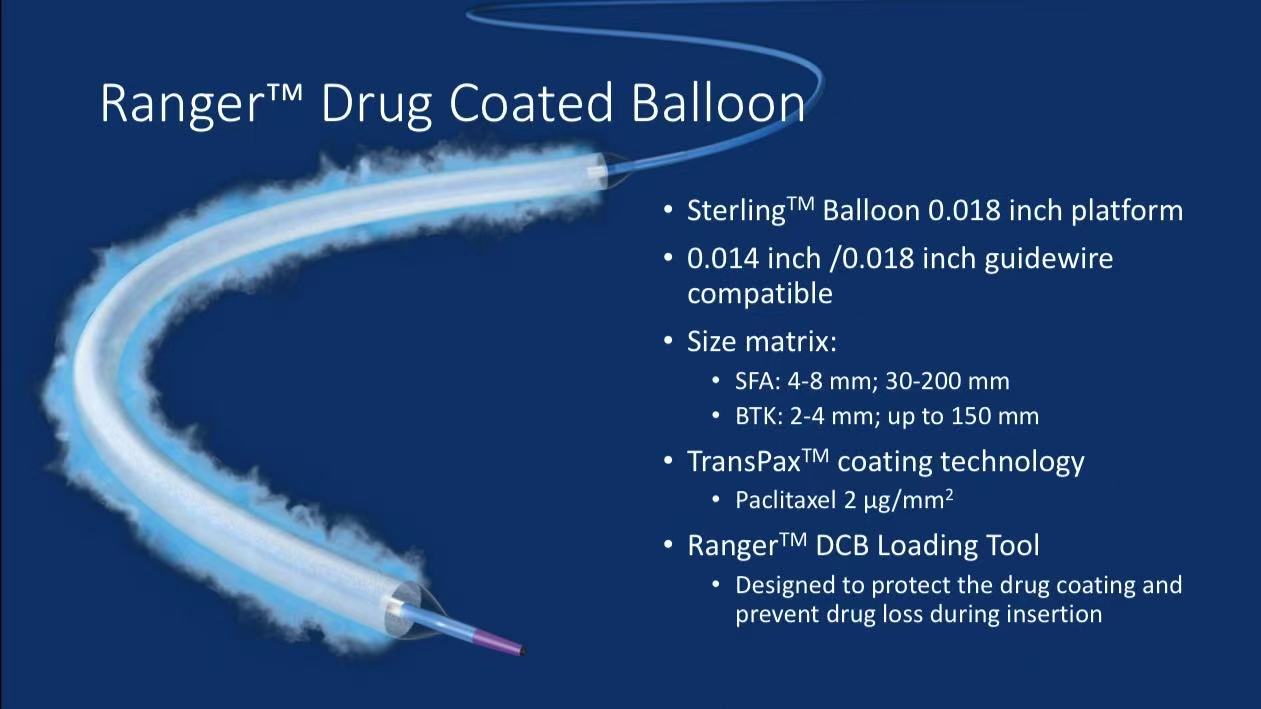
Product Technical Highlights
1.Platform Design:
0.018-inch Sterling™ balloon platform compatible with 0.014/0.018-inch guidewires
Comprehensive size matrix (SFA: 4-8mm diameter, 30-200mm length; BTK: 2-4mm diameter, ≤150mm length)
2.Drug Coating System:
TransPax™ proprietary coating ensures precise 2µg/mm² paclitaxel dose
Unique loading tool minimizes drug loss during delivery
3.Clinical Validation Advantages:
Demonstrated 84.2% 1-year freedom from CD-TLR in ISR lesions (ELEGANCE subgroup)
Special penetration capability in calcified lesions (PACSS 3-4 accounted for 47.8%)
Conclusions and Clinical Implications
1.Ranger™ DCB provides a durable and effective treatment option for femoropopliteal artery disease, with 5-year outcomes significantly superior to conventional PTA
2.Maintains consistent efficacy in high-risk patients (diabetes, long lesions, calcified lesions), expanding DCB application boundaries
3.Safety data alleviates concerns about paclitaxel-related mortality increase, providing confidence for clinical use
4.Product design balances versatility and specificity, suitable for both routine lesions and complex anatomical challenges