Speaker: Osamu Iida, MD, PhD
Hospital: Cardiovascular Division, Osaka Police Hospital, Japan
Abstract
This prospective multicenter study evaluated the 5-year efficacy and safety of the GORE® VIABAHN® Endoprosthesis in 321 patients (324 limbs) with complex femoropopliteal lesions (86.6% TASC C/D). Results demonstrated 62.4% primary patency, 75.9% freedom from TLR, and 0% acute limb ischemia (ALI) at 5 years, with no stent fractures, supporting its durability in long-segment disease.
Introduction
Long-segment femoropopliteal occlusions (≥10 cm) pose significant challenges. The VIABAHN® device, featuring ePTFE and heparin coating (CBAS®), aims to improve long-term outcomes. This post-market study provides real-world evidence from Japan.
Case Analysis
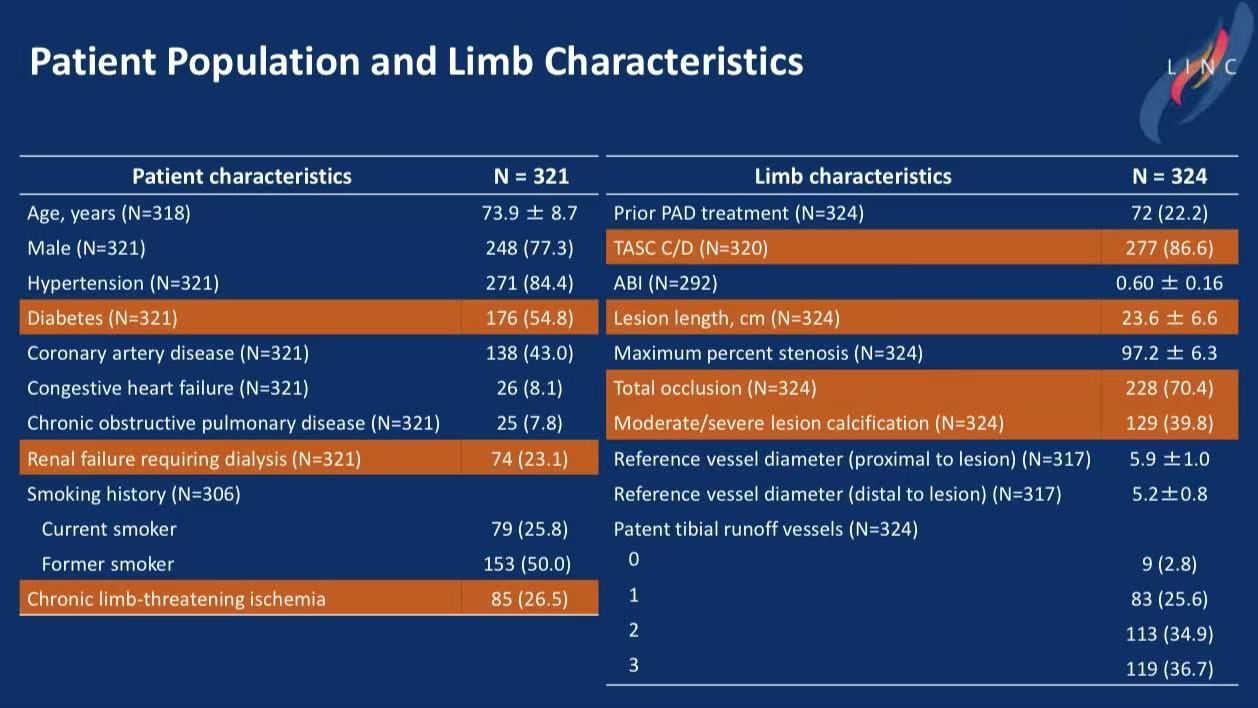
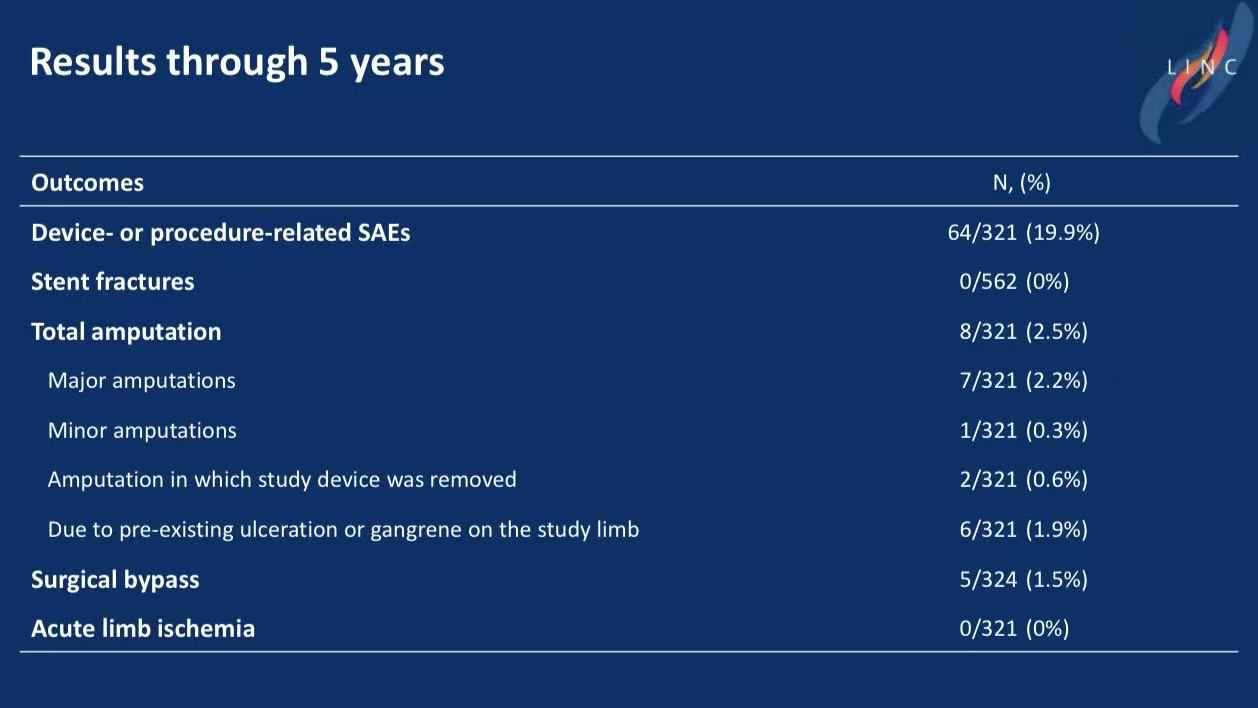
Study Design: Prospective, 64 centers, 5-year follow-up. Mean lesion length 23.6 cm, 70.4% total occlusions.


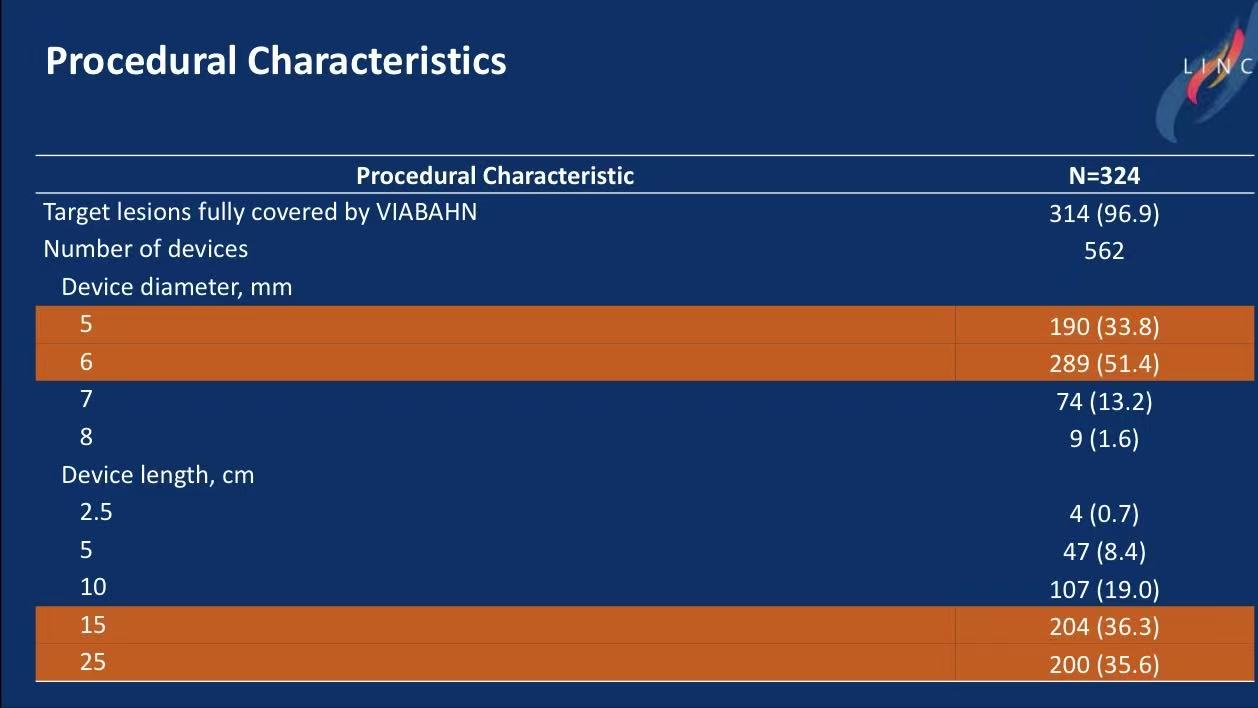
Procedure: 96.9% lesions fully covered; mean 1.7 stents per limb (common size: 6×250 mm).
Key Outcomes:
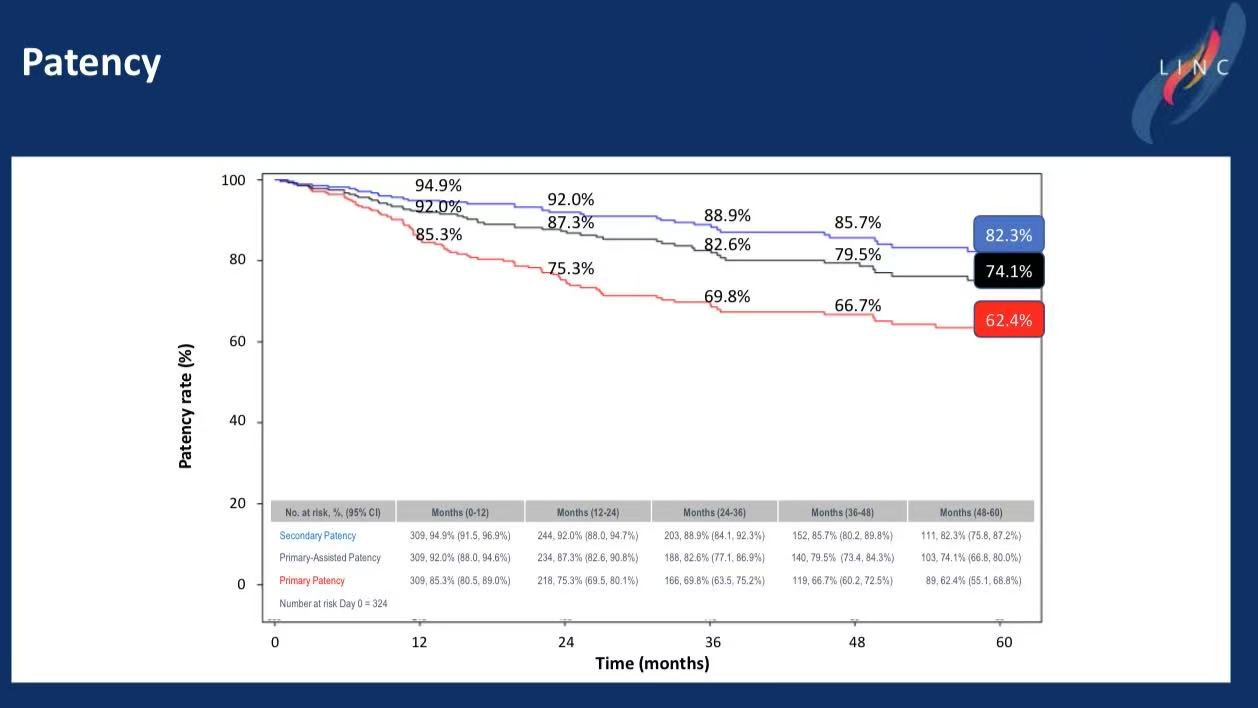
Patency: 5-year primary patency 62.4%, secondary patency 82.3%.
Safety: 19.9% device-related SAEs (mainly restenosis); zero stent fractures.
Clinical Improvement: 89.8% achieved Rutherford class ≤2 at 5 years.

Product Highlights
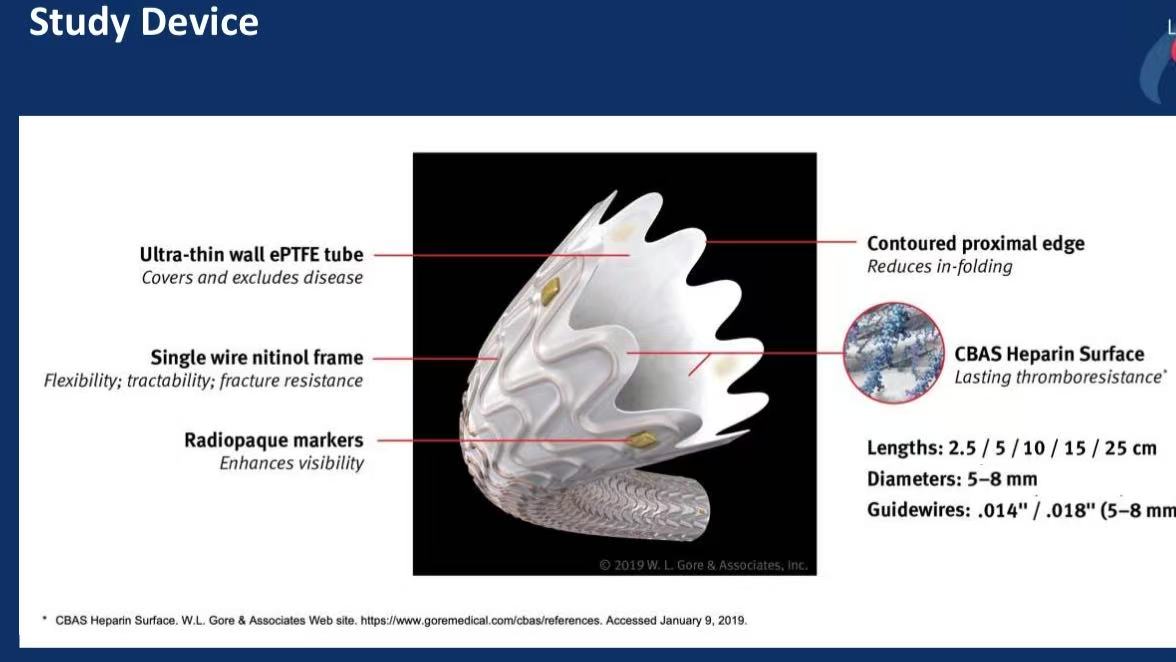
GORE® VIABAHN® Endoprosthesis:
Material: Ultra-thin ePTFE with nitinol frame for flexibility and fracture resistance.
Design: Contoured proximal edge minimizes infolding; CBAS® heparin surface reduces thrombosis.
Indications: Ideal for long (≥10 cm), calcified, or occluded femoropopliteal lesions.
Advantages: Sustained patency in high-risk cohorts (diabetes: 54.8%; dialysis: 23.1%).
Conclusion
1.Efficacy: VIABAHN® outperforms DCBs and bare-metal stents in 5-year primary patency for complex lesions.
2.Safety: Zero ALI and stent fractures highlight mechanical reliability.
3.Versatility: Consistent outcomes across calcification severity and lesion lengths support broad applicability.



