Speaker: Yann Goueffic, MD, PhD
Hospital: Department of Vascular and Endovascular Surgery, Groupe Hospitalier Paris Saint Joseph, Paris, France
Abstract
This study evaluated the 3-year safety outcomes of the IN.PACT Admiral drug-coated balloon (DCB) in diabetic and non-diabetic patients with femoropopliteal peripheral artery disease (PAD) using the French National Health Data System (SNDS). Results showed significantly higher rates of major amputation (7.9% vs. 2.5%), all-cause mortality (25.5% vs. 16.1%), and total reintervention (48.1% vs. 40.6%) in diabetic patients, underscoring diabetes as a critical risk factor in PAD management.
Introduction
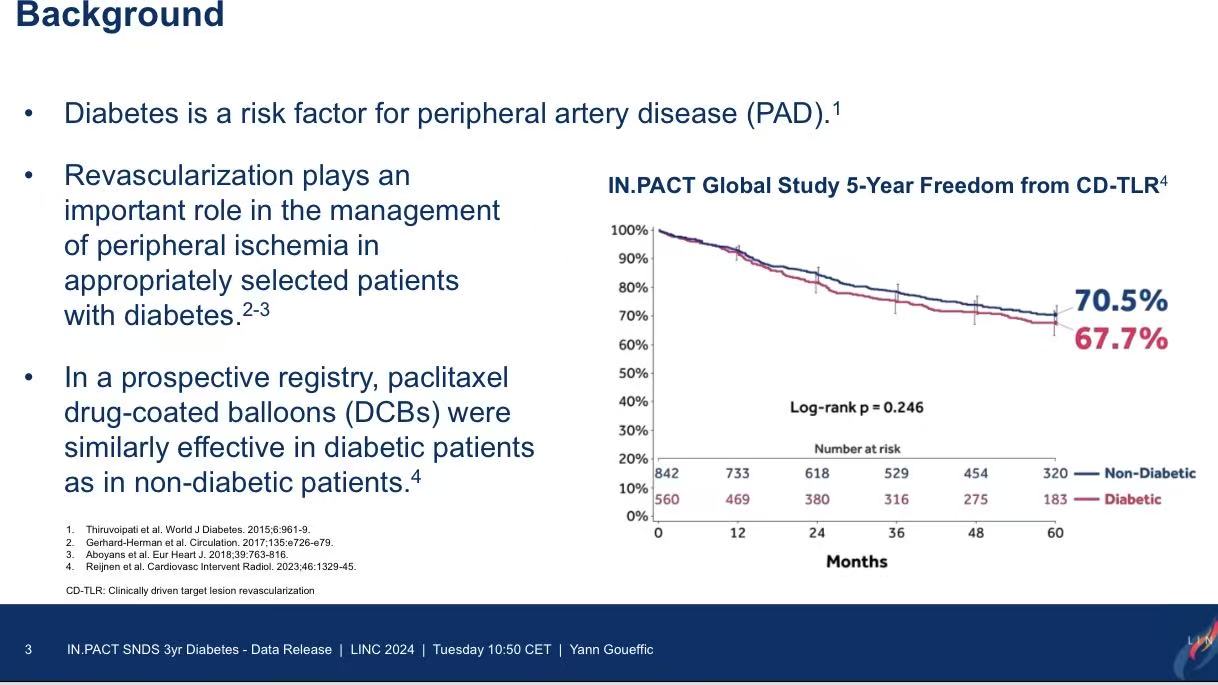
Diabetes is a major risk factor for PAD, and DCBs have emerged as a key tool for femoropopliteal revascularization. However, prior trials and registries faced limitations such as selection bias. This nationwide cohort study provides comprehensive real-world data on long-term outcomes.
Case Analysis
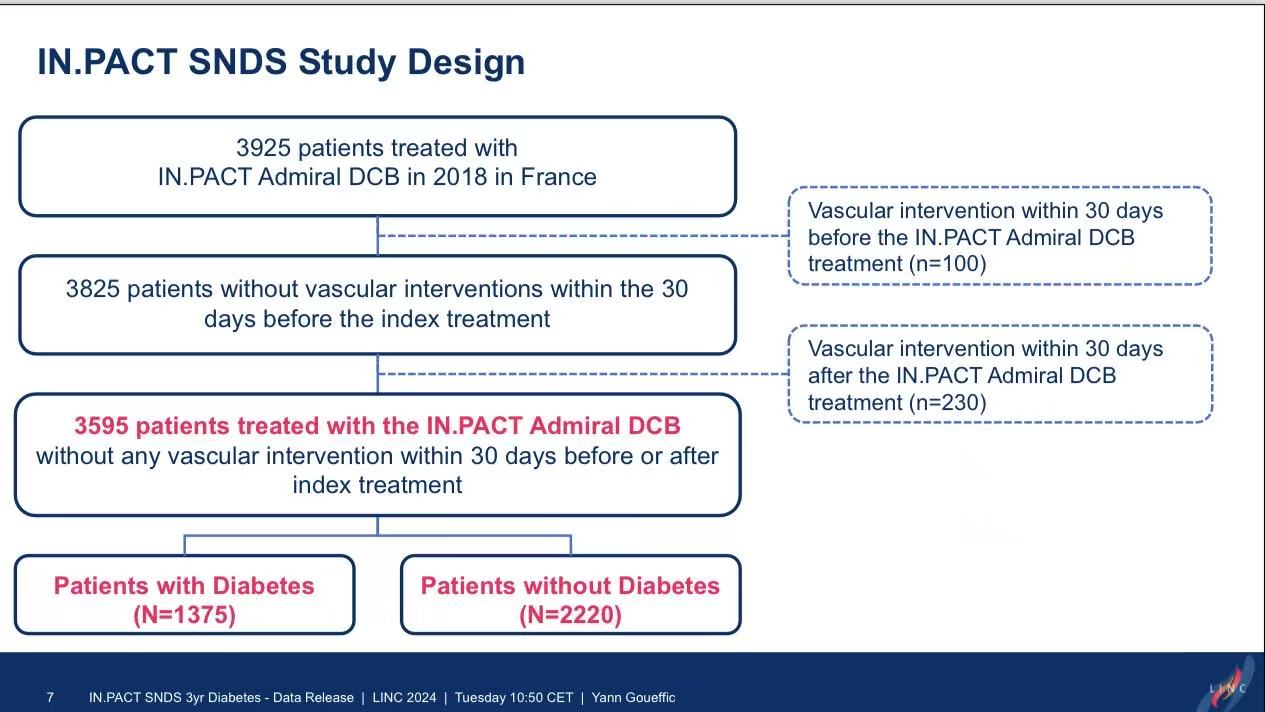
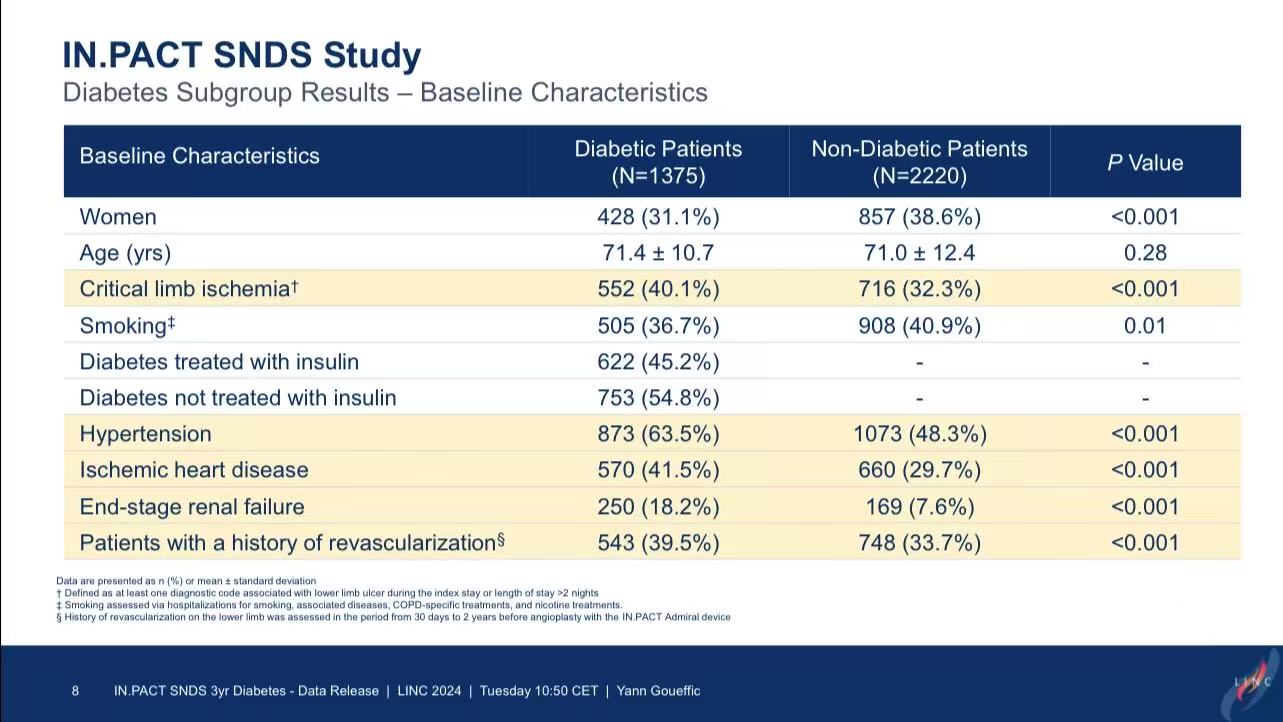
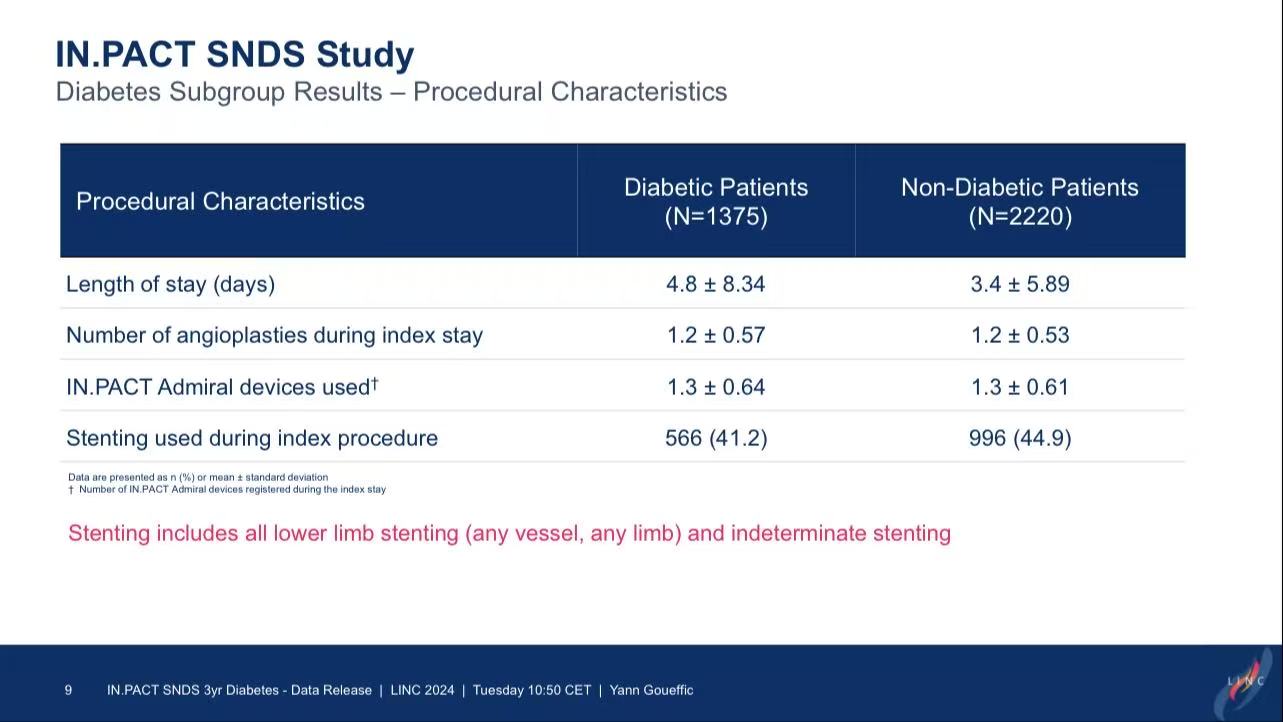
Study Design: Included 3,595 patients treated with IN.PACT Admiral DCB in 2018 (1,375 diabetic vs. 2,220 non-diabetic).
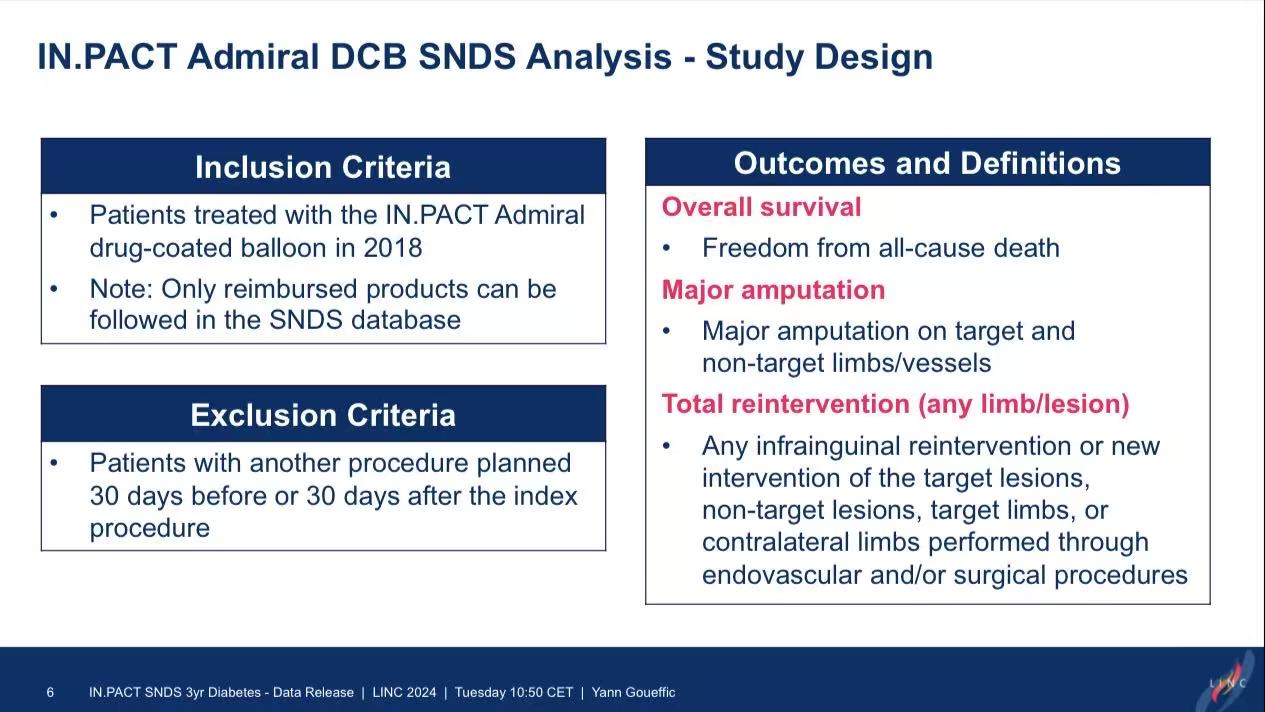
Endpoints: Major amputation, all-cause mortality, and reintervention.
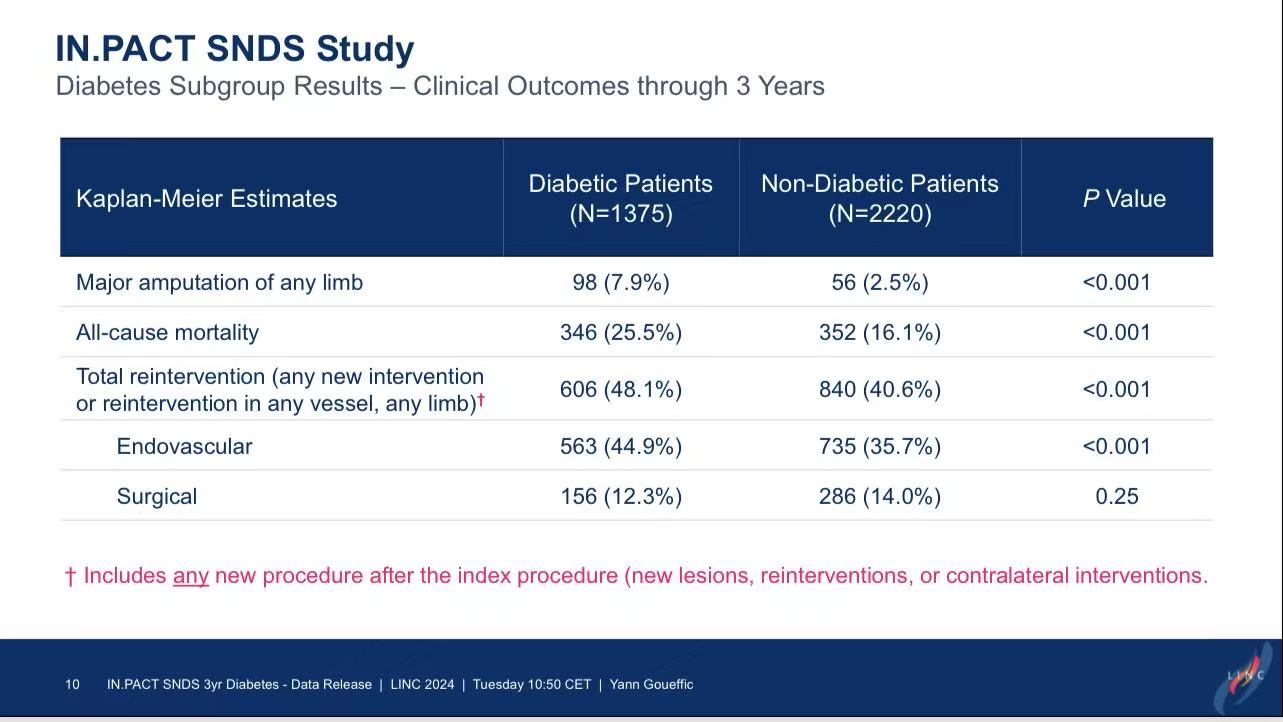
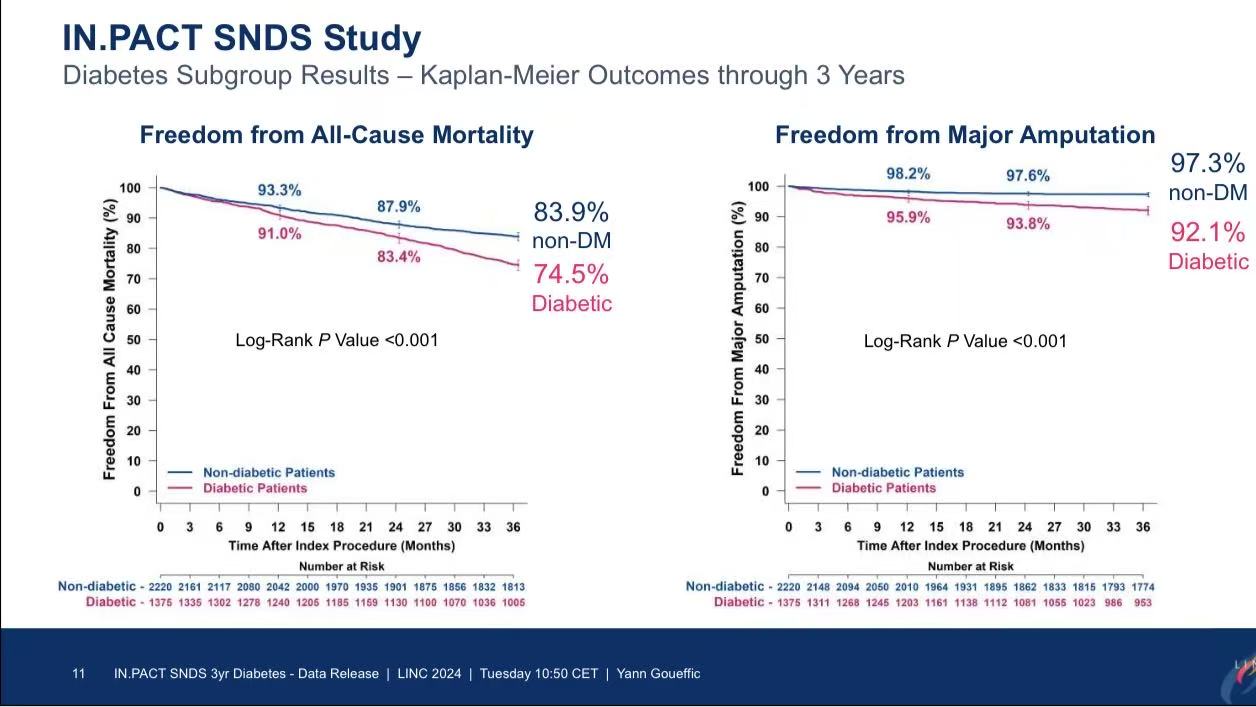
Key Findings:
1.Diabetic patients had triple the risk of major amputation (7.9% vs. 2.5%, P<0.001).
2.All-cause mortality was significantly higher in diabetics (25.5% vs. 16.1%, P<0.001).
3.Higher reintervention rates in diabetics (48.1% vs. 40.6%, P<0.001) may reflect disease progression.
Product Highlights
IN.PACT Admiral DCB: Paclitaxel-coated balloon reducing restenosis via antiproliferative effects.
Indications: Ideal for long-segment lesions or in-stent restenosis in femoropopliteal arteries.
Advantages: Demonstrated safety in real-world settings, but long-term risks in diabetics require vigilance.
Conclusion
1.While the IN.PACT Admiral DCB shows stable performance in real-world practice, diabetic patients face significantly higher risks of amputation, mortality, and reintervention.
2.Personalized management and comprehensive diabetic care are essential.



