
VEITH symposium is one of the most authoritative, large-scale and long-standing academic events in vascular surgery in the world. Thousands of top experts from vascular surgery, interventional radiology, interventional cardiac surgery, and other vascular surgery fields from around the world attend the VEITH symposium conference each year. Here brings together various research data updates from the world, very pioneering, prospective and controversial topic discussion, clinical technology sharing with great application value, the latest and cutting-edge medical devices and technology exploration, face-to-face communication opportunities for world-class authoritative vascular surgery experts, and so on.
The 50th Annual Symposium on Vascular and Endovascular Issues (VEITH 2023) meeting was held at the Hilton Hotel, New York Center, USA from November 14 to 18, 2023. Vascular surgery experts from all over the world actively attended the meeting and brought wonderful speeches. Here, we will sort out the key academic contents of VEITH 2023, and the following are the key views shared by overseas experts. Welcome to read.
New expansion of Supera stent application field

Rajiv Parakh
MEDANTA THE MEDICITY MEDICAL CENTER, GUERGON, INDIA
Since August 2015, 365 patients with non-femoropopliteal disease underwent Supera stent implantation at MEDANTA THE MEDICITY Medical Center in Gurgon, India, including 360 aortoiliac lesions, 48 subclavian lesions, and 11 lesions at other sites. There were no acute occlusions requiring intervention 30 days post-procedure. Five years after surgery, the aortoiliac artery patency was 80.2% and the subclavian artery patency was 82%. This set of data remains in follow-up and more results are expected.
A prospective, multicenter, real-world registry assessed the efficacy, safety, and durability of Supera stenting for de novo or restenotic SFA or complex femoropopliteal lesions in India. The study included 276 patients at 21 centers with diabetes mellitus 84%, hypertension 63%, CAD 30%, mean lesion length 105.4 mm, diameter stenosis 91.7%, occlusion 60%, Rutherford grades 5 and 6 53.2%, and TASC grades C and D 53%. A total of 324 Supera stents were implanted; freedom from TLR was 90.3% at 18 months and 92% at 12 months with Rutherford clinical improvement, and patients' quality of life was also significantly improved.
Supera vascular bionic design provides a more effective solution for complex anatomical environments, conforming to the native vascular anatomy and movement direction while also providing sufficient radial support and flexibility, and is a sharp tool for the treatment of complex superficial femoral artery, femoropopliteal artery, and even common femoral artery lesions. At present, the expanded application of Supera stent in the treatment of arterial lesions at other sites has been carried out, and preliminary progress has been made in the treatment of anastomotic lesions of subclavian artery, aortoiliac artery and AVF. More expanded applications and results are expected to be published.
Experience with the InCraft ® AAA Stent System

Giovanni Torsello
Berlin Medical University
The associated clinical study of the InCraft ® AAA Stent System used during the procedure, the INNOVATION study [1], was a multicenter, open-label, prospective, nonrandomized study. The objective was to assess the technical success and safety of InCraft ® in patients with abdominal aortic aneurysms. From Table 1, it can be seen that both 4 and 5 year follow-up results were excellent. However, because the included patients were strictly screened, it may have deviated from the actual clinical situation. Therefore, the INSIGHT study, a real-world clinical study of the product, was subsequently carried out.
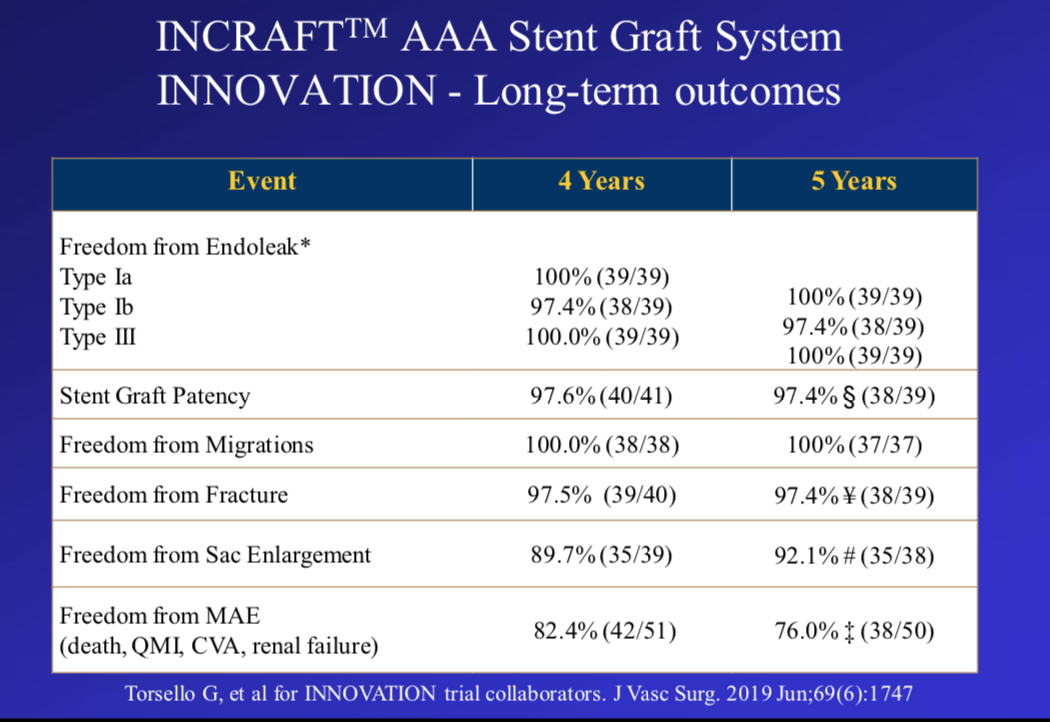
Table 1. Excellent 4-year and 5-year follow-up results of INNOVATION study
Real World INSIGHT Study
The INSIGHT study [2] included 150 patients from 23 medical centers across Europe. Thirty-seven patients (24.7%) had aortic neck < 15 mm, 18.3% had moderately severe iliac tortuosity, and the mean iliac minimum crossing diameter was 7.6 mm (range, 3.4-11.6). Freedom from type Ia endoleak was 93.3% at 3 years of follow-up. Freedom from AAA enlargement was 96.5%.
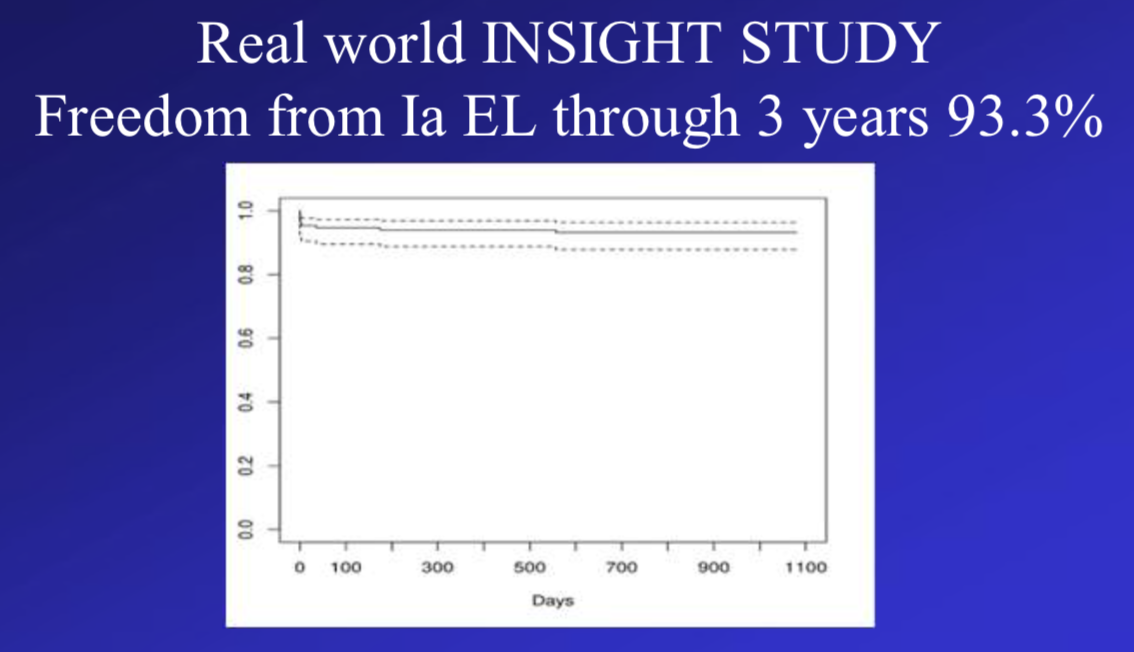
Freedom from type Ia endoleak through 3 years of follow-up
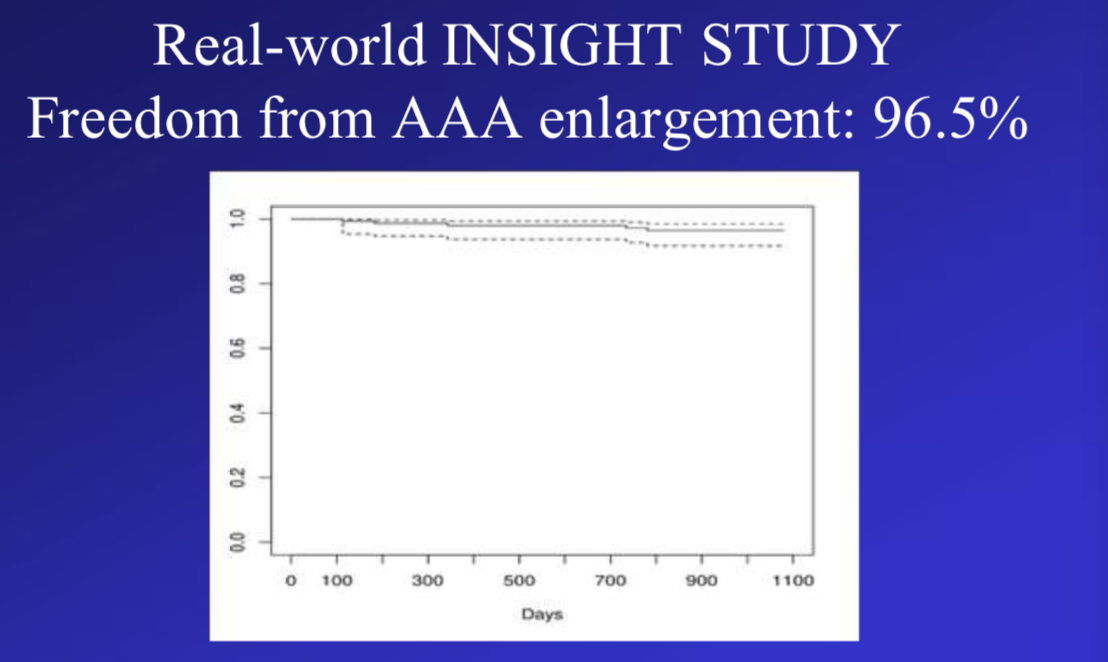
Freedom from AAA enlargement
Clinical Experience with Supera Stent and Recent Data from Related Studies

Chris Metzger
OhioHealth River Methodist Hospital
Unique implantation mode of Supera stent
Three key steps for correct implantation: pre-dilate the lesion segment according to the reference vessel diameter, so that its diameter is ≥ stent diameter; select the stent diameter according to the vessel diameter 1:1; magnify the imaging system to observe the stent mesh geometry and slowly release it.
In preconditioning, aggressive vessel preparation (≥ 1:1) was required; all vessel segments covered by the Supera stent were preconditioned to ensure complete balloon expansion (and if not, a shorter NC PTA was added); PTA under roadmap was clearly sized; and individuals preferred the pressure-focused balloon.
After adequate vessel preparation, stent implantation can begin. Definite distal stent positioning under roadmap; once started, need to zoom in and release slowly; right hand release, left hand guard and adjust. Decelerate and apply forward pressure if stretching too much; decelerate and apply reverse pressure if compressing too much. If this is the first stent implanted, expand the stent and consider more aggressive vessel preparation prior to placement of the next stent.
Advantages and disadvantages of Supera stent
Supera stents can be used in heavily calcified SFA/popliteal lesions; stents are placed in the popliteal artery or adductor canal; stents are used in long lesions; most SFA lesions without ostia are stented; and all CFA or popliteal arteries require stents.
Supera stents have a minimum diameter of 4.5 mm, so thinner vessels may not be available; during vessel preparation, if the lesion cannot be fully dilated, it may affect the stent implantation effect; when there is a large mismatch in the size of the vessel covered by a single stent.
Two-year results of the SWING trial of sirolimus DCB in BTK occlusive disease

Ramon Varcoe
Prince of Wales Hospital
The SWING trial was a prospective, multicenter, single-arm feasibility study that included 35 patients at 8 centers in Australia, New Zealand, and Europe to assess the safety and efficacy of Sundance sirolimus DCB for the treatment of infrapopliteal arterial occlusive disease.
Inclusion criteria Infrapopliteal stenotic or occluded lesions were treated with Sundance DCB, with reference vessel diameter of 2 – 4 mm and total lesion length ≤ 230 mm. Postoperative follow-up was 36 months.
The primary safety endpoint was defined as the number of patients free of major limb adverse events (MALE) and perioperative mortality at 30 days post-procedure.
The primary efficacy endpoint was defined as the rate of late lumen loss assessed by quantitative angiography at 6 months post-procedure. Both primary endpoints of the SWING trial have been met.
Two-year safety and efficacy data from the SWING trial suggest that sirolimus DCB has promising promise in the treatment of below-knee lesions in patients with chronic limb threatening ischemia (CLTI) who currently have limited and challenging treatment options and are expected to continue to improve patient outcomes.






