Author: Dr. D. Chris Metzger
Institution: OhioHealth Riverside Methodist Hospital, Ohio, USA
Summary
This presentation summarizes the one-year follow-up data from the C-GUARDIANS pivotal IDE trial, evaluating the safety and efficacy of the CGuard MicroNet stent in carotid artery stenting (CAS). The CGuard MicroNet stent is designed to reduce perioperative microembolization risk. Trial data demonstrate extremely low rates of stroke, death, and myocardial infarction (MI) over both short and intermediate periods, validating its neuroprotective benefits.
Trial Design and Primary Endpoints
• Design: C-GUARDIANS is a prospective, multicenter, single-arm international trial involving 316 high-risk patients across 24 sites in the United States and Europe. Of these patients, 24.3% were symptomatic. The trial aimed to assess the CGuard stent’s performance in preventing DSMI (death, stroke, myocardial infarction).
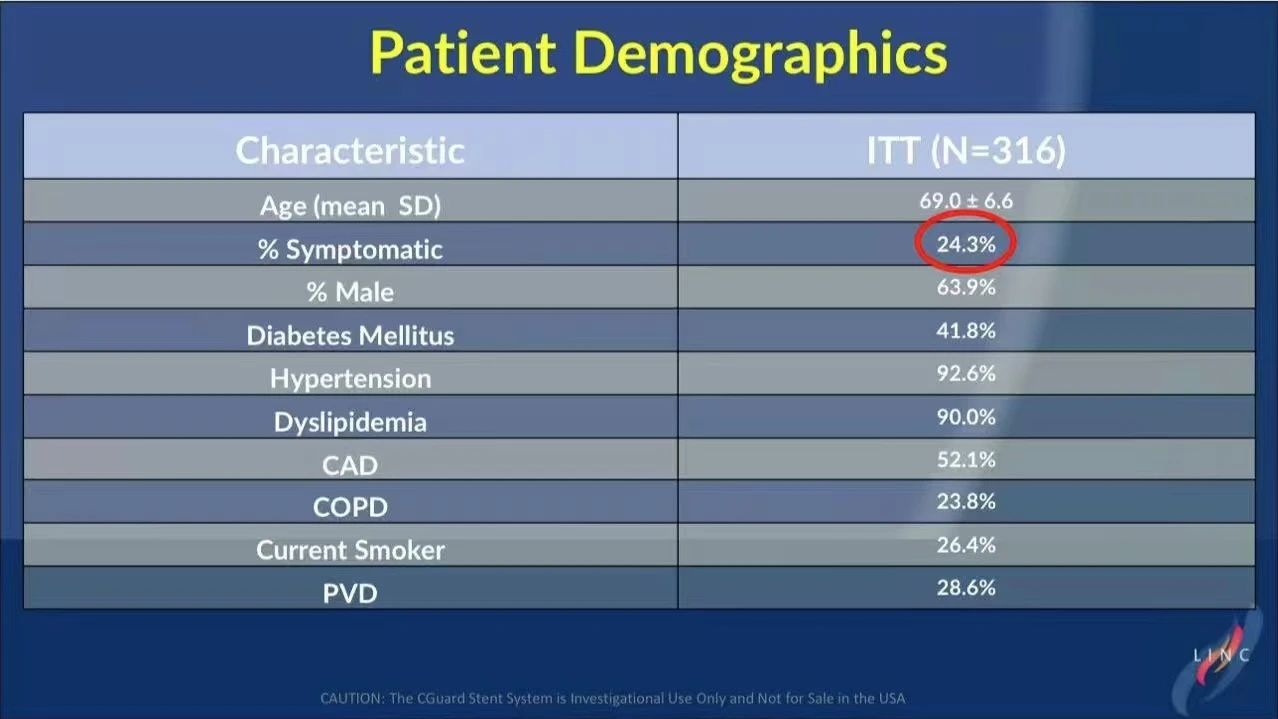
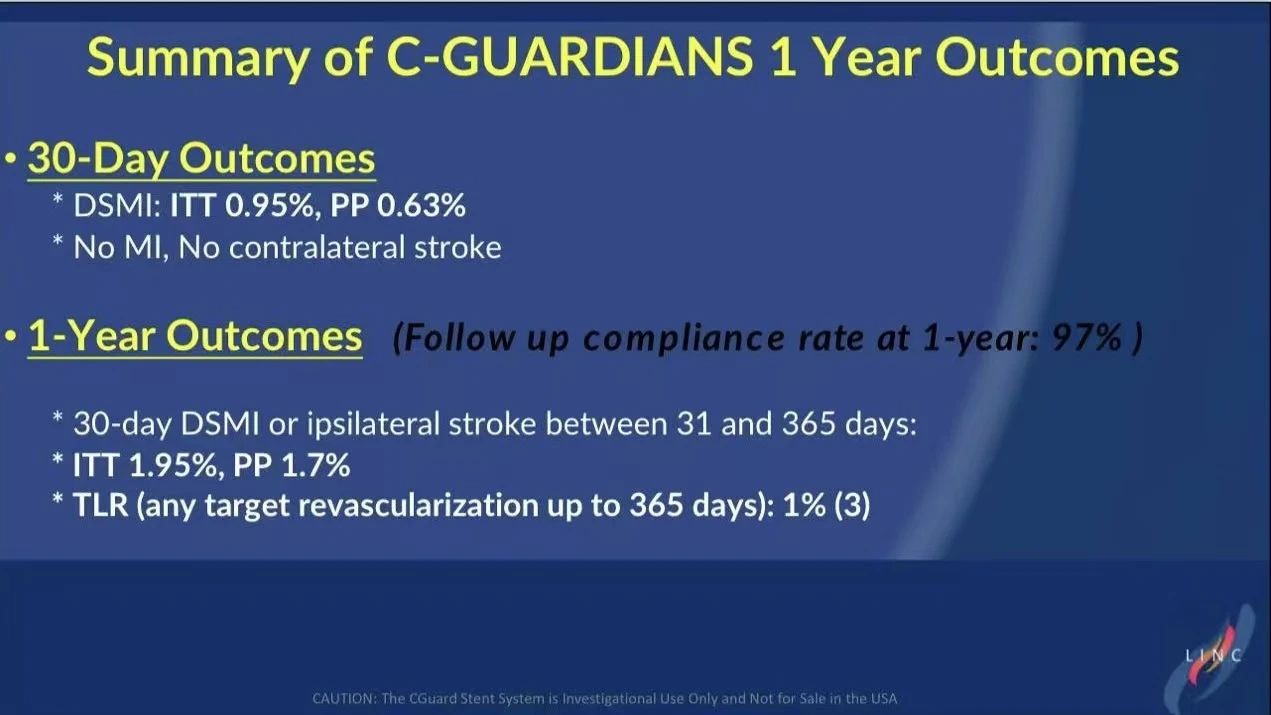
• Primary Endpoints: The primary endpoints included DSMI events within 30 days and ipsilateral stroke incidence within one year. The results showed a 30-day DSMI rate of just 0.95% and a one-year ipsilateral stroke rate of 1.95%.
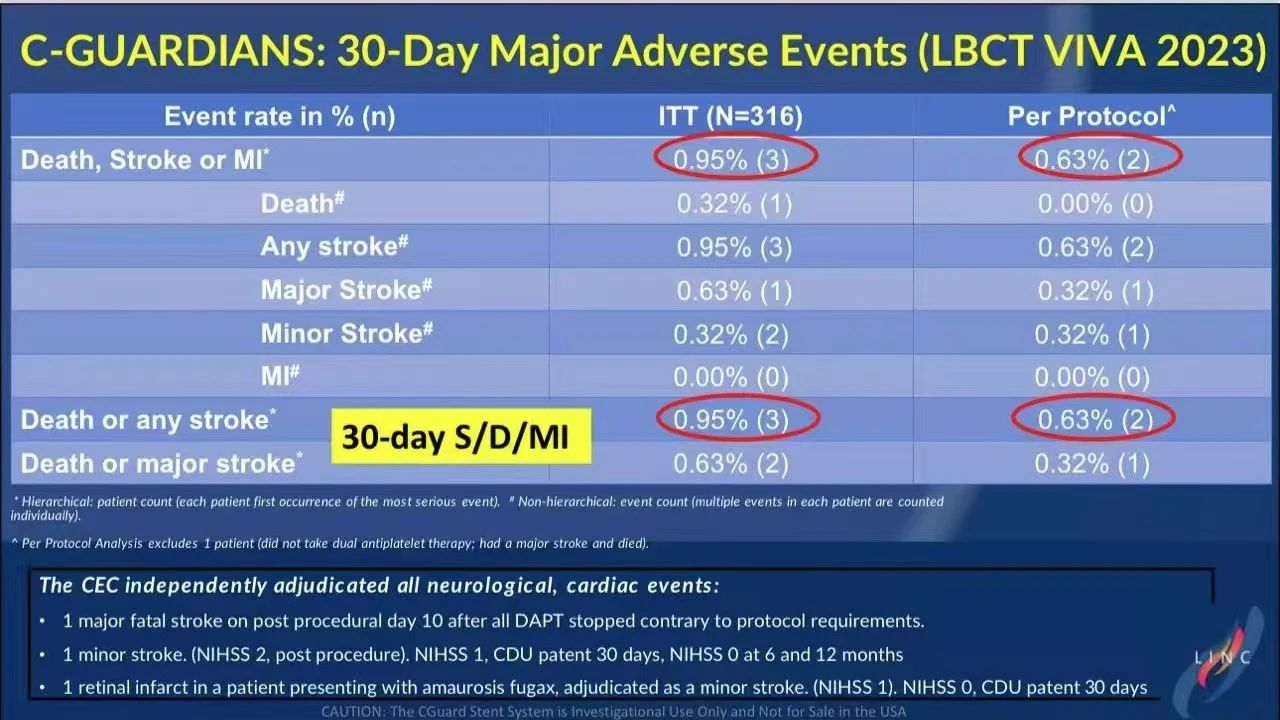
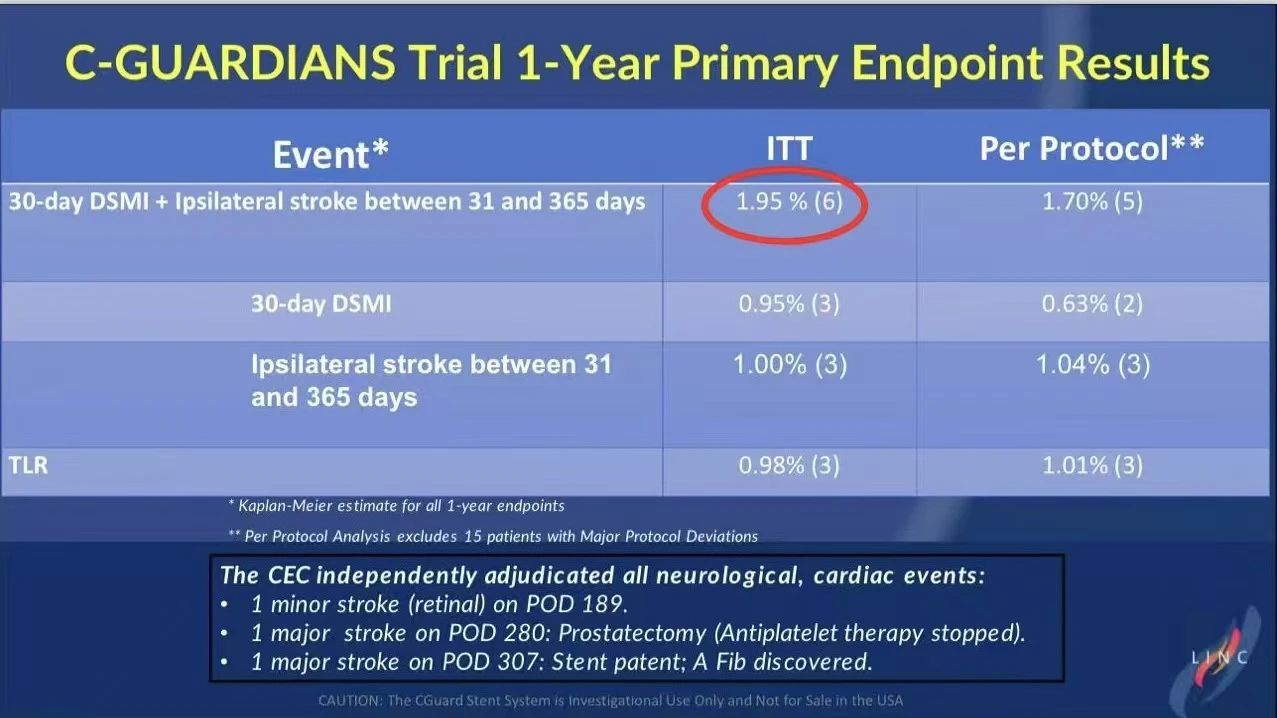
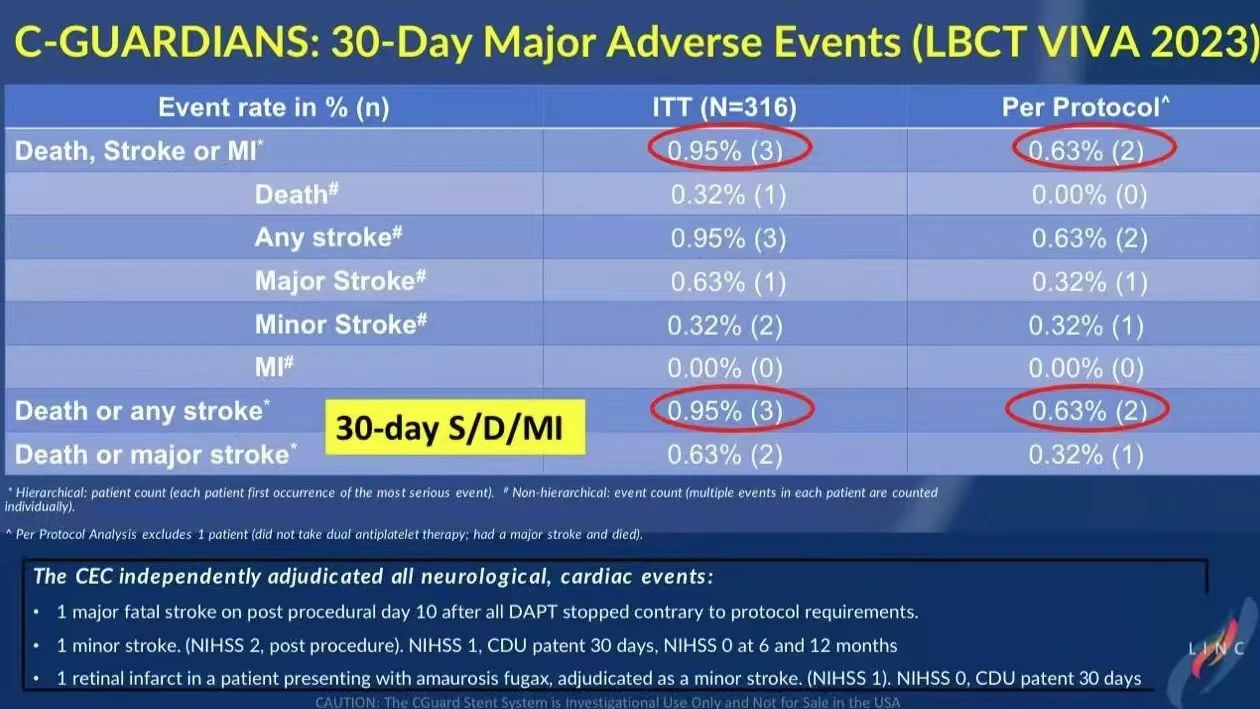
30-Day and One-Year Follow-Up Data
• 30-Day Results: No MI events were observed within 30 days. The DSMI rate was 0.95% in the intent-to-treat (ITT) group and 0.63% in the per-protocol (PP) group, with no cases of contralateral stroke, indicating excellent short-term safety for the CGuard stent.
• One-Year Results: The cumulative DSMI or ipsilateral stroke event rate at one year was 1.95% in the ITT group and 1.7% in the PP group, supporting the CGuard stent’s long-term safety as a viable choice for CAS.
Neuroprotective Benefits of the CGuard MicroNet Stent
• Advantages of MicroNet Technology: The CGuard stent utilizes MicroNet technology with a high-density mesh structure that stabilizes plaque and thrombotic materials, preventing their entry into the bloodstream and reducing microembolization risk. Trial data indicate that the CGuard stent significantly lowers stroke incidence compared to traditional open-cell stents.

Conclusion
1. The C-GUARDIANS trial demonstrates that the CGuard MicroNet stent exhibits extremely low DSMI rates in CAS, indicating robust safety and neuroprotective benefits.
2. MicroNet technology effectively reduces perioperative microembolization events, making it well-suited for neuroprotection in high-risk CAS patients.
3. The trial data support the CGuard stent as a frontline option for carotid revascularization, particularly for patients who require maximum embolization risk reduction.

Contact Us
• Email: endovascluar@simtomax.cn
The English content has been synchronized with the Vasco Knight account. For more international information, please visit:
• Facebook: Vasco Knight
• Instagram: knight_vasco
Let’s safeguard health together and showcase your brilliance to the world!






